Enol ether
In organic chemistry an enol ether is an alkene with an alkoxy substituent.[1] The general structure is R2C=CR-OR where R = H, alkyl, or aryl. A common subfamily of enol ethers are vinyl ethers, with the formula ROCH=CH2. Important enol ethers include the reagent 3,4-dihydropyran and the monomers methyl vinyl ether and ethyl vinyl ether.
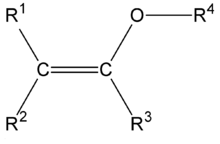
Reactions and uses
Akin to enamines, enol ethers are electron-rich alkenes by virtue of the electron-donation from the heteroatom via pi-bonding. Enol ethers have oxonium ion character. By virtue of their bonding situation, enol ethers display distinctive reactivity. In comparison with simple alkenes, enol ethers exhibit enhanced susceptibility to attack by electrophiles such as Bronsted acids. Similarly, they undergo inverse demand Diels-Alder reactions.[2]
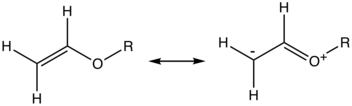
The reactivity of enol ethers is highly dependent on the presence of substituents alpha to oxygen. The vinyl ethers are susceptible to polymerization to give polyvinyl ethers.[3] Some vinyl ethers also find some use as inhalation anesthetics. Enol ethers bearing α substituents do not polymerize readily. They are mainly of academic interest, e.g. as intermediates in the synthesis of more complex molecules.
Preparation
Although enol ethers can be considered the ether of the corresponding enolates, they are not prepared by alkylation of enolates. Some enol ethers are prepared from saturated ethers by elimination reactions.[4]
Alternatively, vinyl ethers can be prepared by transesterification of vinyl esters, especially the widely available vinyl acetate:[5]
- ROH + CH2=CHOAc → ROCH=CH2 + HOAc
Vinyl ethers can be prepared by reaction of acetylene and alcohols in presence of a base.[6]
See also
References
- Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012). Organic Chemistry (2nd ed.). Oxford University Press. p. 295. ISBN 978-0-19-927029-3.
- Percy S. Manchand (2001). "Ethyl Vinyl Ether". eEROS. doi:10.1002/047084289X.re125.
- Gerd Schröder (2012). "Poly(Vinyl Ethers)". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_011.CS1 maint: uses authors parameter (link)
- Carl Kaiser and Joseph Weinstock (1976). "Alkenes Via Hofmann Elimination: Use of Ion-exchange Resin for Preparation of Quaternary Ammonium Hydroxides: Diphenylmethyl Vinyl Ether". Org. Synth. 55: 3. doi:10.15227/orgsyn.055.0003.CS1 maint: uses authors parameter (link)
- Tomotaka Hirabayashi, Satoshi Sakaguchi, Yasutaka Ishii (2005). "Iridium-catalyzed Synthesis of Vinyl Ethers from Alcohols and Vinyl Acetate". Org. Synth. 82: 55. doi:10.15227/orgsyn.082.0055.CS1 maint: uses authors parameter (link)
- Ernst Hofmann, Hans‐Joachim Klimisch, René Backes, Regina Vogelsang, Lothar Franz, Robert Feuerhake (2011). "Vinyl Ethers". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_435.pub.CS1 maint: uses authors parameter (link)