Distonic ion
Distonic ions are chemical species that contain two ionic charges on the same molecule separated by two or more carbon or heteroatoms.[1] Distonic radical ions are unique due to the fact that its charges and radical sites are in different locations (on separate atoms ), unlike regular radicals where the formal charge and unpaired electron are in the same location. These molecular species are created when ionization takes place with either zwitterions or diradicals; ultimately, a neutral molecule loses an electron.[2] Through experimental research distonic radicals have been found to be extremely stable gas phase ions[3] and can be separated into different classes depending on the inherent features of the charged portion of the ion.[4]
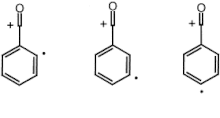
History
In 1984 scientists Bouma, Radom and Yates originated the term through extensive experimental research but they were not the first to deal with distonic ions. Experiments date back to the 1970s with Gross and McLafferty who were the first to propose the idea of such a species.[5]
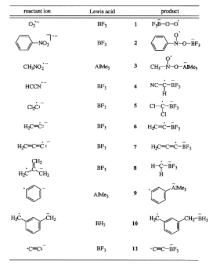
Ion structure
There are several efficient techniques in place that can detect the presence of distonic ions, however when deciding on which method is most appropriate, one must keep in mind the ions internal energy and lifespan.[3] Collisions between ions and neutrally charged molecules allow an individual to detect the location of the radical and charge site in order to ensure the ion is indeed not just a regular radical ion.[7] When a molecule is ionized and can structurally be classified as a distonic ion, the molecules kinetics and thermodynamic properties have been greatly altered. However, additional chemical properties are based on the reactions of the central excited ions. Mass spectrometry techniques are used to study their chemistry.[8]
Experimental data
Distonic ions have been extensively examined due to their unique behavior and how common they can occur.[2] Through the obtained data, it has been shown that in most cases distonic ions have corresponding bonding arrangement as the original molecule before ionization occurred. However data has also shown that distonic ions possess less stability than before ionization occurred; having said that, this does not take away from the fact that distonic ions are considered stable ions and have caught many scientist attention because they possess more stability than its traditional isomer. [3]Challenges do arise when trying to decipher the functions of the charge and radical site because distonic ions are limited to elementary reactions such as unimolecular reactions involving highly excited and short-lived species. [9]
References
- Muller, P. (1994). "Glossary of terms used in physical organic chemistry (IUPAC Recommendations 1994)". Pure and Applied Chemistry. 66 (5): 1077–1184. doi:10.1351/pac199466051077. ISSN 1365-3075.
- Tomazela, Daniela Maria; Sabino, Adão A.; Sparrapan, Regina; Gozzo, Fabio C.; Eberlin, Marcos N. (July 2006). "Distonoid ions". Journal of the American Society for Mass Spectrometry. 17 (7): 1014–1022. doi:10.1016/j.jasms.2006.03.008. PMID 16713292.
- Stirk, Krista M.; Kiminkinen, L. K. Marjatta; Kenttamaa, Hilkka I. (November 1992). "Ion-molecule reactions of distonic radical cations". Chemical Reviews. 92 (7): 1649–1665. doi:10.1021/cr00015a008.
- Hill, Brian T.; Poutsma, John C.; Chyall, Leonard J.; Hu, Jun; Squires, Robert R. (September 1999). "Distonic ions of the 'ate' class". Journal of the American Society for Mass Spectrometry. 10 (9): 896–906. doi:10.1016/S1044-0305(99)00053-7.
- Williams, Peggy E.; Jankiewicz, Bartlomiej J.; Yang, Linan; Kenttamaa, Hilkka I. (12 November 2013). "ChemInform Abstract: Properties and Reactivity of Gaseous Distonic Radical Ions with Aryl Radical Sites". ChemInform. 44 (46): no. doi:10.1002/chin.201346233.
- Hill, Brian T.; Poutsma, John C.; Chyall, Leonard J.; Hu, Jun; Squires, Robert R. (September 1999). "Distonic ions of the "Ate" class". Journal of the American Society for Mass Spectrometry. 10 (9): 896–906. doi:10.1016/S1044-0305(99)00053-7.
- Yu, Sophia J.; Holliman, Christopher L.; Rempel, Don L.; Gross, Michael L. (1993-10-01). "The .beta.-distonic ion from the reaction of pyridine radical cation and ethene: a demonstration of high-pressure trapping in Fourier transform mass spectrometry". Journal of the American Chemical Society. 115 (21): 9676–9682. doi:10.1021/ja00074a037. ISSN 0002-7863.
- Holman, R.W.; Rozeboom, M.D.; Gross, M.L.; Warner, C.D. (January 1986). "Mass spectrometry for investigations of gas-phase radical cation chemistry". Tetrahedron. 42 (22): 6235–6244. doi:10.1016/S0040-4020(01)88085-6.
- Stirk, Krista G.; Kenttamaa, Hilkka I. (1991-07-01). "Radical type reactivity in a .gamma.-distonic radical cation: a gas-phase experimental study". Journal of the American Chemical Society. 113 (15): 5880–5881. doi:10.1021/ja00015a062. ISSN 0002-7863.
- "Distonic radical cations : Guidelines for the assessment of their stability". Tetrahedron. 42 (22): 6225–6234. January 1986. doi:10.1016/S0040-4020(01)88084-4.
- Stirk, Krista M.; Orlowski, Joseph C.; Leeck, Diane T.; Kenttamaa, H. I. (October 1992). "The identification of distonic radical cations on the basis of a reaction with dimethyl disulfide". Journal of the American Chemical Society. 114 (22): 8604–8606. doi:10.1021/ja00048a038. ISSN 0002-7863.
- Hammerum, Steen (1988). "Distonic radical cations in gaseous and condensed phase". Mass Spectrometry Reviews. 7 (2): 123–202. Bibcode:1988MSRv....7..123H. doi:10.1002/mas.1280070202. ISSN 1098-2787.
- Bjoernholm, Thomas.; Hammerum, Steen.; Kuck, Dietmar. (1988-06-01). "Distonic ions as reacting species". Journal of the American Chemical Society. 110 (12): 3862–3869. doi:10.1021/ja00220a023. ISSN 0002-7863.