Diethyl phosphorochloridate
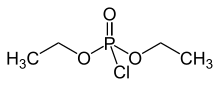
Diethyl chlorophosphate is an organophosphorus compound with the formula (C2H5O)2P(O)Cl. As an reagent in organic synthesis, it is use the convert alcohols to the corresponding diethylphosphate esters. It is a colorless liquid with a fruity odor. It is a corrosive, and as a cholinesterase inhibitor, highly toxic through dermal absorption.[1] The molecule is tetrahedral.
 | |
| Names | |
|---|---|
| IUPAC name
1-[chloro(ethoxy)phosphoryl]oxyethane | |
| Other names
diethylchlorophosphate; Diethoxyphosphorus oxychloride; Diethyl chlorophosphonate; Diethyl phosphorochloride; Diethoxyphosphoryl chloride; O,O-Diethyl chlorophosphate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.011.270 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H10ClO3P | |
| Molar mass | 172.54 g/mol |
| Appearance | white solid |
| Density | 1.1915 g/cm3 |
| Boiling point | 60 °C (140 °F; 333 K) (2 mm Hg) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis and reactions
The compound is prepared by the chlorination of diethylphosphite with carbon tetrachloride (Atherton–Todd reaction).[2]
The compound is electrophilic. Controlled hydrolysis gives tetraethyl pyrophosphate. Alcohols react to vie mixed phosphate esters: [3]
- (C2H5O)2P(O)Cl + ROH → (C2H5O)2P(O)OR + HCl
The reagent is routinely employed in organic synthesis for phosphorylation of carboxylates,[4] alcohols,[5] and amines.[6]
See also
- Diethyl chlorophosphate at www.chemicalbook.com.
References
- "Haz-Map Category Details". hazmap.nlm.nih.gov. Retrieved 2016-07-30.
- Steinberg, Geo. M. (1950). "Reactions of Dialkyl Phosphites. Synthesis of Dialkyl Chlorophosphates, Tetraalkyl Pyrophosphates, and Mixed Orthophosphate Esters". Journal of Organic Chemistry. 15: 637–47. doi:10.1021/jo01149a031.
- Young, Jonathan R. (2001). "Diethyl phosphorochloridate". e-EROS Encyclopedia of Reagents for Organic Synthesis: 1–3.
- Michael A. Insalaco, D. Stanley Tarbell (1970). "tert-Butyl Azidoformate". Org. Synth. 50: 9. doi:10.15227/orgsyn.050.0009.CS1 maint: uses authors parameter (link)
- D. C. Muchmore (1972). "Preparation and Reductive Cleavage of Enol Phosphates: 5-Methylcoprost-3-ene". Org. Synth. 52: 109. doi:10.15227/orgsyn.052.0109.
- Nick Nikolaides, Ioanna Schipor, Bruce Ganem (1995). "Conversion of Amines to Phospho Esters: Decyl Diethyl Phosphate". Org. Synth. 72: 246. doi:10.15227/orgsyn.072.0246.CS1 maint: uses authors parameter (link)