Dichlorotetrakis(pyridine)rhodium(III) chloride
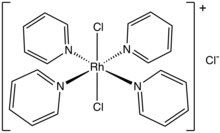
Dichlorotetrakis(pyridine)rhodium(III) chloride is the chloride salt of the coordination complex with the formula [RhCl2(pyridine)4]+. It is a yellow solid that crystallizes from water as the tetrahydrate,[1] which converts to the monohydrate upon vacuum drying at 100 °C. It is prepared by treating rhodium trichloride with an excess of pyridine in the presence of a catalytic amount of a reductant.[2]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
| C20H20Cl3N4Rh | |
| Molar mass | 525.66 g·mol−1 |
| Appearance | yellow solid |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H315, H319, H335 |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Related complexes
- [RhCl3(pyridine)3] is an intermediate in the synthesis of [RhCl2(pyridine)4]Cl.[3]
References
- Vasilchenko, D. B.; Baidina, I. A.; Filatov, E. Yu.; Korenev, S. V. (2009). "Structure and thermal properties of [RhPy4Cl2]X complex salts (X = Cl−, ReO 4 − , ClO 4 − )". Journal of Structural Chemistry. 50 (2): 335–342. doi:10.1007/s10947-009-0046-7.
- Gillard, R..; Wilkinson, G. W. (1939). "Trans -Dichlorotetra(pyridine)Rhodium(III) Salts". trans-Dichlorotetra(pyridine)rhodium(III) Salts. Inorganic Syntheses. 1o. pp. 64–67. doi:10.1002/9780470132418.ch11. ISBN 9780470132418.
- Acharya, K. R.; Tavale, S. S.; Guru Row, T. N. (1984). "Structure of mer-trichlorotris(pyridine)rhodium(III), [RhCl3(C5H5N)3]". Acta Crystallographica Section C Crystal Structure Communications. 40 (8): 1327–1328. doi:10.1107/S0108270184007848.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.