Dehydrogenation of amine-boranes
Dehydrogenation of amine-boranes or dehydrocoupling of amine-boranes is a chemical process in main group and organometallic chemistry wherein dihydrogen is released by the coupling of two or more amine-borane adducts. This process gained some interests due to the potential of using amine-boranes for hydrogen storage.
Catalysis
Many metal complexes catalyze the dehydrogenation of amine-borane (AB). Catalysis in the absence of metals has also been observed.[1]
Pathways
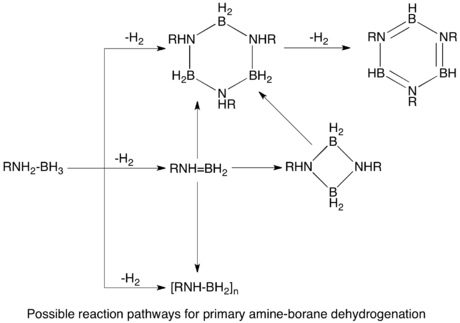
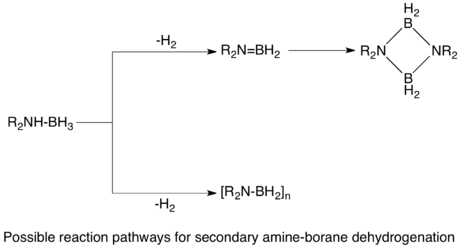
The dehydrogenation of AB would in principle afford (H2BNH2)n and (HBNH)n. The monomers (n = 1) are highly unstable with respect to oligomerization.
Metal carbonyl catalysts
Group 6 metal carbonyls upon photolytic activation catalyze dehydrogenation of AB.[2] Secondary amine-boranes dehydrogenate to form cyclic dimers, or monomeric aminoboranes in the case of more bulky groups on the amine. Similarly, primary amine-boranes dehydrogenate through a two step intramolecular process to give aminoborane polymers, which further dehydrogenate to form borazines.[2] [CpFe(CO)2]2 is also an effective precatalyst, requiring photolytic activation. The two step process is proposed to occur first by dehydrogenation of the amine-borane coordinated to the metal, followed by cyclodimerization in an off-metal step.[1]
Rhodium and iridium catalysts
The first catalysts for the dehydrogenation of ABs were derived from reduction of Rh(I) complexes to form the active colloidal heterogeneous catalyst.[1] As in the case with the metal carbonyl catalysts, bulky secondary amine-boranes form monomeric aminoboranes. For RhL2- and Rh(H)2L2-derived catalysts, the active species is a homogeneous catalyst, with the phosphine ligands interacting directly with the dehydrocoupling process.[3] Changing the phosphine ligands from PiPr3 to PiBu3 significantly increases the turnover rate of the catalyst.[3] Unlike other Rh(I) catalysts, the rhodium analogue of Wilkinson's catalyst RhCl(PHCy2)3 (Cy=cyclohexyl) behaves like the RhL2 and Rh(H)2L2 catalysts as a homogeneous species.[1]
In comparison to RhCl(PHCy2)3, the iridium analogue has reduced catalytic activity on the dehydrogenation of non sterically hindered amine-boranes, and increased activity on more sterically hindered substrates.[1] Dehydrocoupling of primary diborazanes NH2R—BH2—NHR—BH3 can be catalyzed by Brookhart's catalyst via conversion to the metal-bound species MeNH—BH2 and subsequent polymerization/oligomerization. This same reaction has been found to occur in the absence of the iridium metal, upon heating of the reaction mixture.[1] Dehydrogenation of ammonia-borane with Brookhart's catalyst results in quantitative formation of the cyclic pentamer [NH2BH2]5 rather than the typically seen cyclic dimers from other amine-borane dehydrogenations.[4] When catalyzing ammonia-borane dehydrogenation, the catalyst acts homogeneously at a 0.5 mol% catalyst loading.[4] Rather than the typical high temperatures needed for this dehydrogenation, the reaction proceeds cleanly at room temperature, with complete substrate conversion in 14min.[4]
Metallocenes
Group 4 metallocenes also catalyze dehydrogenation of ABs. Activity is affected by metal (Ti > Zr > Hf) and inhibited by bulk. Unlike other catalytic processes, the reaction proceeds via a linear aminoborane [NR2BH2]2, which then cyclodimerizes through a dehydrocoupling process on the metal.[1] Most of the zirconocene complexes contain the zirconium in the +4 oxidation state, and the systems are not very active catalysts for amine-borane dehydrogenation.[5] In contrast to these systems, the cationic zirconocene complex [Cp2ZrOC6H4P(tBu)2]+ effectively catalyzes the reaction, with the most notable example being the dehydrogenation of dimethylamineborane in 10min at room temperature.[5]
Potential applications
Hydrogen storage
Dehydrogenation of amine-boranes is thermodynamically favourable, making the process attractive for hydrogen storage systems. Ammonia borane has attracted particular interest due to its high weight percent of hydrogen (19.6%).[6][7] Dehydrogenation occurs in three steps, creating polyamino-boranes and borazines as insoluble side products.[6][7] The dehydrogenation reactions are irreversible, which limits the utility of this process for hydrogen storage.
Hydrogen transfer
Amine-borane dehydrogenation can be coupled with hydride transfer to unsaturated functional groups, usually olefins in an anti-Markovnikov fashion.[8][9] Hydroboration of the olefin and release of H2 from the amine-borane occur in parallel reactions, reducing the percent of olefin reduced.[8]
References
- Staubitz, A.; Robertson, A. P. M.; Manners, I., "Ammonia-Borane and Related Compounds as Dihydrogen Sources", Chemical Reviews 2010, volume 110, pp. 4079–4124.. doi:10.1021/cr100088b
- Kawano, Y.; Uruichi, M.; Shimoi, M.; Taki, S.; Kawaguchi, T.; Kakizawa, T.; Ogino, H. "Dehydrocoupling Reactions of Borane-Secondary and -Primary Amine Adducts Catalyzed by Group-6 Carbonyl Complexes: Formation of Aminoboranes and Borazines" J. Amer. Chem. Soc. 2009, 131, 14946-14957. doi:10.1021/ja904918u
- Chaplin, A.B.; Weller, A.S. "Amine- and Dimeric Amino-Borane Complexes of the {Rh(PiPr3)2}+ Fragment and their Relevance to the Transition-Metal-Mediated Dehydrocoupling of Amine-Boranes" Inorg. Chem. 2010, 49, 1111–1121. doi:10.1021/ic9020542
- Denney, M.C.; Pons, V.; Hebden, T.J.; Heinekey, D.M.; Goldberg, K.I. "Efficient Catalysis of Ammonia Borane Dehydrogenation" J. Amer. Chem. Soc. 2006, 128, 12048-12049. doi:10.1021/ja062419g
- Chapman, A.M.; Haddow, M.F.; Wass, D.F. "Frustrated Lewis Pairs beyond the Main Group: Cationic Zirconocene-Phosphinoaryloxide Complexes and Their Application in Catalytic Dehydrogenation of Amine Boranes" J. Amer. Chem. Soc. 2011, 133, 8826–8829. doi:10.1021/ja201989c
- Frueh, S.; Kellett, R.; Mallery, C.; Molter, T.; Willis, W.S.; King'ondu, C.; Suib, S.L. "Pyrolytic Decomposition of Ammonia Borane to Boron Nitride" Inorg. Chem. 2011, 50, 783–792. doi:10.1021/ic101020k
- Mal, S.S.; Stephens, F.H.; Baker, R.T. "Transition metal catalyzed dehydrogenation of fuel blends." Chem. Commun. 2011,47,2922–2924. doi:10.1039/c0cc03585h
- Sewell, L.J.; Chaplin, A.B.; Weller, A.S. "Hydroboration of an alkene by amine-boranes catalyzed by a [Rh(PR3)2]+ fragment. Mechanistic insight and tandem hydroboration/dehydrogenation" Dalton Trans. 2011, 40, 7499–7501. doi:10.1039/C1DT10819K
- Couturier, M.; Andresen, B.M.; Tucker, J.L.; Dubé, P.; Brenek, S.J.; Negri, J.T. "The use of borane-amine adducts as versatile palladium-catalyzed hydrogen-transfer reagents in methanol" Chem. Commun. 2001, 42, 2763–2766. doi:10.1016/S0040-4039(01)00300-8
External links
