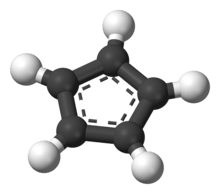
Cyclopentadienyl anion
In chemistry, the cyclopentadienyl anion or cyclopentadienide is an aromatic species with a formula of [C5H5]− and abbreviated as Cp−.[1] Its name derives from the molecule cyclopentadiene.
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| [C5H5]− or Cp− | |||
| Molar mass | 65.09 g/mol | ||
| Conjugate acid | Cyclopentadiene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
It is a regular pentagonal, planar, cyclic ion; as well, it has 6 π-electrons (4n + 2, where n = 1), which fulfills Hückel's rule of aromaticity. It can coordinate as a ligand to metal atoms.
The structure shown is a composite of five resonance contributors in which each carbon atom carries part of the negative charge.
Salts of the cyclopentadienyl anion can be stable, e.g., sodium cyclopentadienide.
Coordination compounds of the cyclopentadienyl anion (not the cyclopentadienyl radical) are known as cyclopentadienyl complexes. Biscyclopentadienyl complexes are called metallocenes.
See also
- Cyclopentadienyl (radical), C5H5
- Cyclopentadienyl complex
- Cyclopentadiene, C5H6
- Cyclooctatetraenide anion, [C8H8]2−
- Metallocene
References
- "Cyclopentadienide". PubChem Compound Database. National Center for Biotechnology Information. Retrieved 14 April 2016.