Circumdatin H
Circumdatin H is an alkaloid. It was isolated, along with related compounds, from the fungus Aspergillus ochraceus.[1]
 | |
| Names | |
|---|---|
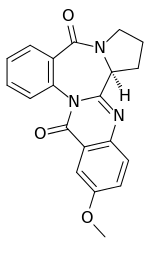
| IUPAC name
(5bS)-2-Methoxy-5b,6,7,8-tetrahydro-10H,16H-pyrrolo[2,1-c]quinazolino[3,2-a][1,4]benzodiazepine-10,16-dione | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C20H17N3O3 | |
| Molar mass | 347.374 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Circumdatin C, and circumdatin F are prototypical members, while other members such as circumdatin D, circumdatin E and circumdatin H have an additional tetrahydropyrrole ring.
The compounds of this group are considered to be useful chemotaxonomic markers. Among these circumdatin H and circumdatin E are able to inhibit the mitochondrial respiratory chain in submitochondrial particles from beef heart, presumable by interfering with NADH oxidase activity (IC50 1.5 μM and 2.5 μM, respectively).
First total synthesis of circumdatin H was reported starting from anthranilic acid.[2]
References
- López-Gresa, M Pilar; González, M Carmen; Primo, Jaime; Moya, Pilar; Romero, Vanessa; Estornell, Ernesto (1 June 2005). "Circumdatin H, a New Inhibitor of Mitochondrial NADH Oxidase, from Aspergillus ochraceus". The Journal of Antibiotics. 58 (6): 416–419. doi:10.1038/ja.2005.54.
- Bose, D.; Chary, M. (2009). "First Total Synthesis of (-)-Circumdatin H, a Novel Mitochondrial NADH Oxidase Inhibitor". Synthesis. 2010 (4): 643. doi:10.1055/s-0029-1218606.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.