Cedrene
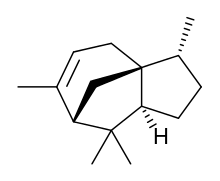
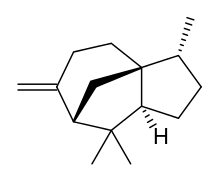
Cedrene is a sesquiterpene found in the essential oil of cedar. The two isomers present in the oil are (−)-α-cedrene[3][4] and (+)-β-cedrene,[5] which differ in the position of a double bond.
 | |
| Names | |
|---|---|
| IUPAC name
(1S,2R,5S,7R)-2,6,6,8-tetramethyltricyclo[5.3.1.01,5]undec-8-ene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.131 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H24 | |
| Molar mass | 204.357 g·mol−1 |
| Density | 0.932 g/mL at 20 °C[1] |
| Boiling point | 261–262 °C[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
 | |
| Names | |
|---|---|
| IUPAC name
(1S,2R,5S,7R)-2,6,6-trimethyl-8-methylidenetricyclo[5.3.1.0(1,5)]undecane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.131 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H24 | |
| Molar mass | 204.357 g·mol−1 |
| Density | 0.932 g/mL at 20 °C[2] |
| Boiling point | 263–264 °C[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
See also
- Cedrol, another component of cedar oil
References
- Sigma-Aldrich Co., (−)-α-Cedrene. Retrieved on 8 July 2012.
- Sigma-Aldrich Co., (+)-β-Cedrene. Retrieved on 8 July 2012.
- Lee, H. Y.; Lee, S.; Kim, D.; Kim, B. K.; Bahn, J. S.; Kim, S. (1998). "Total synthesis of α-cedrene: A new strategy utilizing N-Aziridinylimine radical chemistry". Tetrahedron Letters. 39 (42): 7713–7716. doi:10.1016/S0040-4039(98)01680-3.
- Takigawa, H.; Kubota, H.; Sonohara, H.; Okuda, M.; Tanaka, S.; Fujikura, Y.; Ito, S. (1993). "Novel Allylic Oxidation of alpha-Cedrene to sec-Cedrenol by a Rhodococcus Strain". Applied and Environmental Microbiology. 59 (5): 1336–1341. PMC 182086. PMID 16348930.
- Kerr, W. J.; McLaughlin, M.; Morrison, A. J.; Pauson, P. L. (2001). "Formal total synthesis of (+/-)-alpha- and beta-cedrene by preparation of cedrone. Construction of the tricyclic carbon skeleton by the use of a highly efficient intramolecular Khand annulation". Organic Letters. 3 (19): 2945–2948. doi:10.1021/ol016054a. PMID 11554814.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.