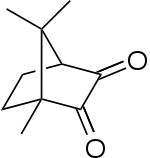
Camphorquinone
Camphorquinone, also known as 2,3-bornanedione, is a photoinitiator used in curing dental composites.[1]
 | |
| Names | |
|---|---|
| IUPAC name
2,6-Bornanedione | |
| Other names
Camphorquinone 6-Oxocamphor CQ CPQ | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.030.728 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C10H14O2 | |
| Molar mass | 166.220 g·mol−1 |
| Melting point | 197-203 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Polymerization is induced very slowly by camphorquinone, so amines such as N,N-dimethyl-p-toluidine, 2-ethyl-dimethylbenzoate, N-phenylglycine are generally added to increase the rate of curing.[1]
It absorbs very weakly at 468 nm (extinction coefficient of 40 M−1·cm−1) giving it a pale yellow color.[1] Photoexcitation results in nearly quantitative formation of its triplet state through intersystem crossing and very faint fluorescence.[2]
It can be hydrolyzed by the enzyme 6-oxocamphor hydrolase.
References
- Jakubiak, J.; Allonas, X.; Fouassier, J.P.; Sionkowska, A.; Andrzejewska, E.; Linden, L.Å.; Rabek, J.F. (August 2003). "Camphorquinone–amines photoinitating systems for the initiation of free radical polymerization". Polymer. 44 (18): 5219–5226. doi:10.1016/S0032-3861(03)00568-8.
- Allonas, Xavier; Fouassier, Jean-Pierre; Angiolini, Luigi; Caretti, Daniele (19 September 2001). "Excited-State Properties of Camphorquinone Based Monomeric and Polymeric Photoinitiators". Helvetica Chimica Acta. 84 (9): 2577. doi:10.1002/1522-2675(20010919)84:9<2577::AID-HLCA2577>3.0.CO;2-Q.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.