cDNA library
A cDNA library is a combination of cloned cDNA (complementary DNA) fragments inserted into a collection of host cells, which constitute some portion of the transcriptome of the organism and are stored as a "library". cDNA is produced from fully transcribed mRNA found in the nucleus and therefore contains only the expressed genes of an organism. Similarly, tissue-specific cDNA libraries can be produced. In eukaryotic cells the mature mRNA is already spliced, hence the cDNA produced lacks introns and can be readily expressed in a bacterial cell. While information in cDNA libraries is a powerful and useful tool since gene products are easily identified, the libraries lack information about enhancers, introns, and other regulatory elements found in a genomic DNA library.
cDNA Library Construction
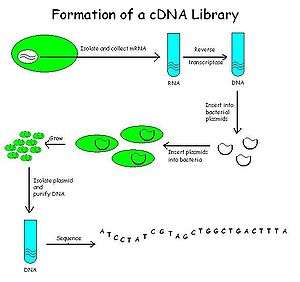
cDNA is created from a mature mRNA from a eukaryotic cell with the use of reverse transcriptase. In eukaryotes, a poly-(A) tail (consisting of a long sequence of adenine nucleotides) distinguishes mRNA from tRNA and rRNA and can therefore be used as a primer site for reverse transcription. This has the problem that not all transcripts, such as those for the histone, encode a poly-A tail.
mRNA extraction
Firstly, the mRNA is obtained and purified from the rest of the RNAs. Several methods exist for purifying RNA such as trizol extraction and column purification. Column purification is done by using oligomeric dT nucleotide coated resins where only the mRNA having the poly-A tail will bind. The rest of the RNAs are eluted out. The mRNA is eluted by using eluting buffer and some heat to separate the mRNA strands from oligo-dT.
cDNA construction
Once mRNA is purified, oligo-dT (a short sequence of deoxy-thymidine nucleotides) is tagged as a complementary primer which binds to the poly-A tail providing a free 3'-OH end that can be extended by reverse transcriptase to create the complementary DNA strand. Now, the mRNA is removed by using a RNAse enzyme leaving a single stranded cDNA (sscDNA). This sscDNA is converted into a double stranded DNA with the help of DNA polymerase. However, for DNA polymerase to synthesize a complementary strand a free 3'-OH end is needed. This is provided by the sscDNA itself by generating a hairpin loop at the 3' end by coiling on itself. The polymerase extends the 3'-OH end and later the loop at 3' end is opened by the scissoring action of S1 nuclease. Restriction endonucleases and DNA ligase are then used to clone the sequences into bacterial plasmids.
The cloned bacteria are then selected, commonly through the use of antibiotic selection. Once selected, stocks of the bacteria are created which can later be grown and sequenced to compile the cDNA library.
cDNA Library uses
cDNA libraries are commonly used when reproducing eukaryotic genomes, as the amount of information is reduced to remove the large numbers of non-coding regions from the library. cDNA libraries are used to express eukaryotic genes in prokaryotes. Prokaryotes do not have introns in their DNA and therefore do not possess any enzymes that can cut it out during transcription process. cDNA does not have introns and therefore can be expressed in prokaryotic cells. cDNA libraries are most useful in reverse genetics where the additional genomic information is of less use. Additionally, cDNA libraries are frequently used in functional cloning to identify genes based on the encoded protein's function. When studying eukaryotic DNA, expression libraries are constructed using complementary DNA (cDNA) to help ensure the insert is truly a gene.[1]
cDNA Library vs. Genomic DNA Library
cDNA library lacks the non-coding and regulatory elements found in genomic DNA. Genomic DNA libraries provide more detailed information about the organism, but are more resource-intensive to generate and keep.
Cloning of cDNA
cDNA molecules can be cloned by using restriction site linkers. Linkers are short, double stranded pieces of DNA (oligodeoxyribonucleotide) about 8 to 12 nucleotide pairs long that include a restriction endonuclease cleavage site e.g. BamHI. Both the cDNA and the linker have blunt ends which can be ligated together using a high concentration of T4 DNA ligase. Then sticky ends are produced in the cDNA molecule by cleaving the cDNA ends (which now have linkers with an incorporated site) with the appropriate endonuclease. A cloning vector (plasmid) is then also cleaved with the appropriate endonuclease. Following "sticky end" ligation of the insert into the vector the resulting recombinant DNA molecule is transferred into E. coli host cell for cloning.
See also
References
- P., Clark, David (2009). Biotechnology : applying the genetic revolution. Pazdernik, Nanette Jean. Amsterdam: Academic Press/Elsevier. ISBN 9780121755522. OCLC 226038060.