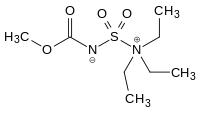
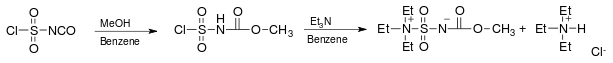Burgess reagent
The Burgess reagent (methyl N-(triethylammoniumsulfonyl)carbamate) is a mild and selective dehydrating reagent often used in organic chemistry.[1][2] It was developed in the laboratory of Edward M. Burgess at Georgia Tech.
 | |
| Names | |
|---|---|
| IUPAC name
1-Methoxy-N-triethylammoniosulfonyl-methanimidate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.157.812 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H18N2O4S | |
| Molar mass | 238.30 g·mol−1 |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H315, H319, H335 |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
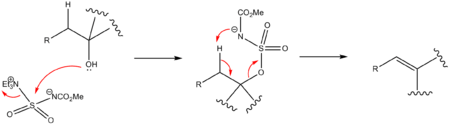
The Burgess reagent is used to convert secondary and tertiary alcohols with an adjacent proton into alkenes. Dehydration of primary alcohols does not work well. The reagent is soluble in common organic solvents and alcohol dehydration takes place with syn elimination through an intramolecular elimination reaction. The Burgess reagent is a carbamate and an inner salt. A general mechanism is shown below.
Preparation
The reagent is prepared from chlorosulfonylisocyanate by reaction with subsequent treatment with methanol and triethylamine in benzene:[3]
References
- Atkins, G. M.; Burgess, E. M. (1968). "The reactions of an N-sulfonylamine inner salt". J. Am. Chem. Soc. 90 (17): 4744–4745. doi:10.1021/ja01019a052.
- Sachin Khapli, Satyajit Dey & Dipakranjan Mal (2001). "Burgess reagent in organic synthesis" (PDF). J. Indian Inst. Sci. 81: 461–476. Archived from the original (PDF) on 2004-03-02.
- Edward M. Burgess; Harold R. Penton Jr. & E. A. Taylor (1973). "Thermal reactions of alkyl N-carbomethoxysulfamate esters". J. Org. Chem. 38 (1): 26–31. doi:10.1021/jo00941a006.