Brusselator
The Brusselator is a theoretical model for a type of autocatalytic reaction. The Brusselator model was proposed by Ilya Prigogine and his collaborators at the Université Libre de Bruxelles.[1] It is a portmanteau of Brussels and oscillator.
It is characterized by the reactions
Under conditions where A and B are in vast excess and can thus can be modeled at constant concentration, the rate equations become
where, for convenience, the rate constants have been set to 1.
The Brusselator has a fixed point at
- .
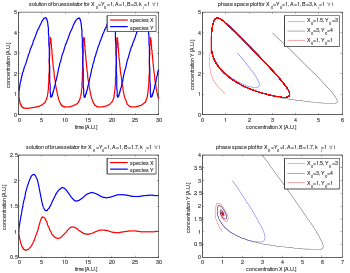
The fixed point becomes unstable when
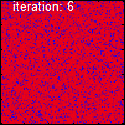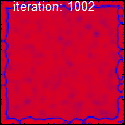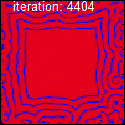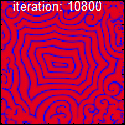
leading to an oscillation of the system. Unlike the Lotka–Volterra equation, the oscillations of the Brusselator do not depend on the amount of reactant present initially. Instead, after sufficient time, the oscillations approach a limit cycle.[2]
The best-known example is the clock reaction, the Belousov–Zhabotinsky reaction (BZ reaction). It can be created with a mixture of potassium bromate , malonic acid , and manganese sulfate prepared in a heated solution of sulfuric acid .[3]
See also
- Lotka–Volterra equation
- Oregonator
References
- "IDEA - Internet Differential Equations Activities". Washington State University. Retrieved 2010-05-16.
- http://www.bibliotecapleyades.net/archivos_pdf/brusselator.pdf Dynamics of the Brusselator
- BZ reaction Archived December 31, 2012, at the Wayback Machine