Bobbitt's salt
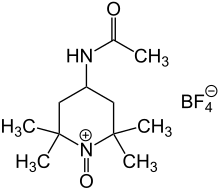
Bobbitt's salt is an oxoammonium compound derived from 4-acetamido-2,2,6,6-tetramethylpiperidine. It contains the tetrafluoroborate anion and is named after the American chemist James M. Bobbitt (born 1930).
 | |
| Names | |
|---|---|
| IUPAC name
N-(2,2,6,6-tetramethyl-1-oxopiperidin-1-ium-4-yl)acetamide;tetrafluoroborate | |
| Other names
4-(Acetylamino)-2,2,6,6-tetramethyl-1-oxo-piperidinium tetrafluoroborate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.202.272 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H21BF4N2O2 | |
| Molar mass | 300.10 g·mol−1 |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H315, H319, H335 |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
As a less expensive analogue of the N-oxoammonium salt derived from TEMPO, Bobbitt's salt is still mainly used as a catalyst for oxoammonium-catalyzed oxidations.[1][2]
(7)

References
- Nabyl Merbouh, James M. Bobbitt, Christian Brückner (2004). "Preparation of Tetramethylpiperdine-1-oxoammonlum Salts and Their Use as Oxidants in Organic Chemistry. A Review". Organic Preparations and Procedures International. 36: 1-31. doi:10.1080/00304940409355369.CS1 maint: uses authors parameter (link)
- James M.Bobbitt, Nicholas A.Eddy, Jay J.Richardson, Stephanie A.Murray, Leon J.Tilley (2013). "Discussion Addendum for: Preparation of 4-Acetylamino-2, 2, 6, 6-tetramethylpiperidine-1-oxoammonium Tetrafluoroborate and the Oxidation of Geraniol to Geranial (2,6-Octadienal, 3,7-dimethyl-, (2e)-)". Org. Synth. 90: 215. doi:10.15227/orgsyn.090.0215.CS1 maint: multiple names: authors list (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.