Blanc chloromethylation
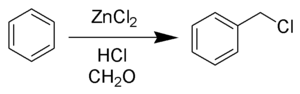
The Blanc chloromethylation (also called the Blanc reaction) is the chemical reaction of aromatic rings with formaldehyde and hydrogen chloride catalyzed by zinc chloride or other Lewis acid to form chloromethyl arenes. The reaction was discovered by Gustave Louis Blanc (1872-1927) in 1923.[1][2][3] The reaction is performed with care as, like most chloromethylation reactions, it produces highly carcinogenic bis(chloromethyl) ether as a by-product.
| Blanc chloromethylation | |
|---|---|
| Named after | Gustave Louis Blanc |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | blanc-reaction |
Mechanism and scope
The reaction is carried out under acidic conditions and with a ZnCl2 catalyst. These conditions protonate the formaldehyde carbonyl making the carbon much more electrophilic. The aldehyde is then attacked by the aromatic pi-electrons, followed by rearomatization of the aromatic ring. The benzyl alcohol thus formed is quickly converted to the chloride under the reaction conditions.
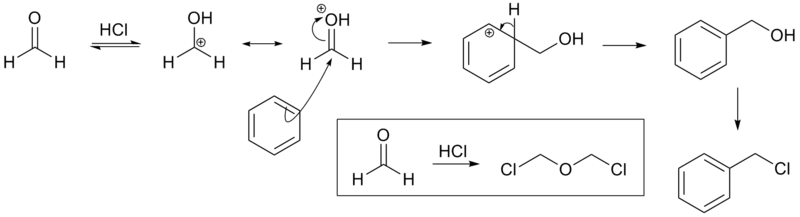
Other possibilities for the electrophile include (chloromethyl)oxonium cation (ClH2C–OH2+) or chlorocarbenium cation (ClCH2+), which may be formed in the presence of zinc chloride.[4] These species may account for the fact that moderately and strongly deactivated substrates that are inert to Friedel-Crafts reactions like acetophenone, nitrobenzene and p-chloronitrobenzene[5] do show marginal reactivity of limited synthetic utility under chloromethylation conditions.[6] Deactivated substrates give better results under modified chloromethylation conditions using chloromethyl methyl ether (MOMCl) in the presence of 60% H2SO4.[4]
Highly activated arenes like phenols and anilines are not suitable substrates, since they undergo further electrophilic attack by Friedel-Crafts alkylation with the formed benzylic alcohol/chloride in an uncontrolled manner. In general, the formation of diarylmethane side product is a common outcome.[6]
Although the reaction is an efficient means of introducing a chloromethyl group, the production of small amounts of highly carcinogenic bis(chloromethyl) ether is a disadvantage for industrial applications.
The corresponding fluoromethylation, bromomethylation and iodomethylation reactions can also be achieved, using the appropriate hydrohalic acid.[7]
Related chloromethylations
Chloromethylation of thiols can be effected with concentrated HCl and formaldehyde:[8]
- ArSH + CH2O + HCl → ArSCH2Cl + H2O
Chloromethylation can also be effected using chloromethyl methyl ether:
- ArH + CH3OCH2Cl → ArCH2Cl + CH3OH
This reaction is employed in the chloromethylation of styrene in the production of ion-exchange resins and Merrifield resins.[9]
See also
- Friedel-Crafts alkylation
- Quelet reaction
- Bouveault–Blanc reduction
References
- Gustave Louis Blanc Bull. Soc. Chim. France 1923, 33, 313
- Whitmore, F. C.; Ginsburg, Abram; Rueggeberg, Walter; Tharp, I.; Nottorf, H.; Cannon, M.; Carnahan, F.; Cryder, D.; FLeming, G.; Goldberg, G.; Haggard, H.; Herr, C.; Hoover, T.; Lovell, H.; Mraz, R.; Noll, C.; Oakwood, T.; Patterson, H.; Van Strien, R.; Walter, R.; Zook, H.; Wagner, R.; Weisgerber, C.; Wilkins, J. (May 1946). "Production of Benzyl Chloride by Chloromethylation of Benzene. Laboratory and Pilot Plant Studies". Industrial & Engineering Chemistry. 38 (5): 478–485. doi:10.1021/ie50437a013.
- Belen'kii, Leonid I; Vol'kenshtein, Yu B; Karmanova, I B (30 September 1977). "New Data on the Chloromethylation of Aromatic and Heteroaromatic Compounds". Russian Chemical Reviews. 46 (9): 891–903. Bibcode:1977RuCRv..46..891B. doi:10.1070/RC1977v046n09ABEH002180.
- Laali, Kenneth K. (2001), "Formaldehyde-Hydrogen Chloride", Formaldehyde–Hydrogen Chloride, Encyclopedia of Reagents for Organic Synthesis, American Cancer Society, doi:10.1002/047084289x.rf022, ISBN 9780470842898
- 研藏, 白川; 泰三, 松川 (1950-01-25). "Chloromethylation of Benzene Nucleus II". Yakugaku Zasshi (in Japanese). 70 (1): 25–28. doi:10.1248/yakushi1947.70.1_25. ISSN 0031-6903.
- McKeever, C. H.; Fuson, Reynold C. (2011-03-15), "Chloromethylation of Aromatic Compounds", Organic Reactions, American Cancer Society, pp. 63–90, doi:10.1002/0471264180.or001.03, ISBN 9780471264187
- C., Norman, Richard O. (2017). Principles of Organic Synthesis, 3rd Edition. Coxon, James M. (3rd ed.). Boca Raton: Routledge. ISBN 9781351421737. OCLC 1042320639.
- D. Enders, S. Von Berg, B. Jandeleit (2002). "Diethyl [(Phenylsulfonyl)methyl]phosphonate". Org. Synth. 78: 169. doi:10.15227/orgsyn.078.0169.CS1 maint: uses authors parameter (link)
- Dardel, François; Arden, Thomas V. (2008). "Ion Exchangers". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a14_393.pub2.