Bistriflimide
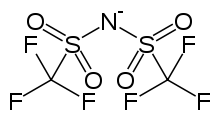
Bistriflimide, systematically known as bis(trifluoromethane)sulfonimide (or 'imidate', see below) and colloquially as TFSI, is a non-coordinating anion with the chemical formula [(CF3SO2)2N]−. Its salts are typically referred to as being metal triflimidates.
Applications
The anion is widely used in ionic liquids (e.g.trioctylmethylammonium bis(trifluoromethylsulfonyl)imide), since it is less toxic and more stable than more "traditional" counterions such as tetrafluoroborate. This anion is also of importance in lithium-ion and lithium metal batteries (LiTFSI) because of its high dissociation and conductivity. It has the added advantage of suppressing crystallinity in poly(ethylene oxide), which increases the conductivity of that polymer below its melting point at 50 °C.
Triflimidic acid
The conjugate acid of bistriflimide, which is frequently referred to by the trivial name triflimidic acid, is a commercially available superacid. It is a crystalline compound, but is hygroscopic to the point of being deliquescent. Owing to its very high acidity and good compatibility with organic solvents it has been employed as a catalyst in a wide range of chemical reactions.[1]
Its pKa value in water cannot be accurately determined but in acetonitrile it has been estimated as −0.10 and in 1,2-dichloroethane −12.3 (relative to the pKa value of 2,4,6-trinitrophenol (picric acid), anchored to zero to crudely approximate the aqueous pKa scale[2]), making it more acidic than triflic acid (pKaMeCN = 0.70, pKaDCE(rel. picric acid) = –11.4).[3]
Naming
Developing an IUPAC name for bistriflimide that indicates the structure and reactivity is challenging, and changes to current names have been proposed. The main difficulty arises from the ambiguous use of the word amide to mean an acylated (including sulfonylated) amine or the anionic form of an amine. Likewise, imide can refer to a bisacylated amine or a twice deprotonated amine. Thus, depending on the system used, there is ambiguity as to whether amide or imide is being used to refer to the parent acid or the anion. (The anion has been referred to as an amidate or imidate in an attempt to distinguish it from the acid.) The complications in naming these compounds was highlighted in an article by the IUPAC.[4] Since then, the IUPAC has recommended (2013) that derivatives of anionic nitrogen can be named as azanides, so bis[(trifluoromethane)sulfonyl]azanide would be an acceptable and unambiguous name for bistriflimide anion. The parent acid, whose trivial name is triflimidic acid, would then be called bis[(trifluoromethane)sulfonyl]azane.[5]
See also
References
- Zhao, Wanxiang; Sun, Jianwei (25 September 2018). "Triflimide (HNTf2) in Organic Synthesis". Chemical Reviews. 118 (20): 10349–10392. doi:10.1021/acs.chemrev.8b00279. PMID 30251840.
- Absolute pKa values in DCE are about 45 units higher, making their values very large for all but the strongest superacids (see: J. Phys. Chem. A 2015, 119, 735).
- Raamat, Elin; Kaupmees, Karl; Ovsjannikov, Gea; Trummal, Aleksander; Kütt, Agnes; Saame, Jaan; Koppel, Ivar; Kaljurand, Ivari; Lipping, Lauri (2012-05-02). "Acidities of strong neutral Brønsted acids in different media". Journal of Physical Organic Chemistry. 26 (2): 162–170. doi:10.1002/poc.2946. ISSN 0894-3230.
- Wilson, Gregory J.; Hollenkamp, Anthony F.; Pandolfo, Anthony G. (July–August 2007). "Resolving Ambiguous Naming for an Ionic Liquid Anion". Chemistry International. 29 (4). Retrieved 2008-01-08.
- Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. Favre, Henri A.,, Powell, Warren H., 1934-, International Union of Pure and Applied Chemistry. Cambridge, England: Royal Society of Chemistry. 2014. ISBN 9781849733069. OCLC 865143943.CS1 maint: others (link)