Benzisothiazolinone
Benzisothiazolinone (BIT) is a widely used biocide and belongs to the group of isothiazolinones.
 | |
| Names | |
|---|---|
| IUPAC name
1,2-benzisothiazol-3(2H)-one | |
| Other names
Benzisothiazolinone, Benzisothiazolin-3-one, Benzisothiazolone | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | BIT |
| 119510 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.018.292 |
| EC Number |
|
| MeSH | 1,2-benzisothiazoline-3-one |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H5NOS | |
| Molar mass | 151.18 g·mol−1 |
| Appearance | white powder |
| Melting point | 158 °C (316 °F; 431 K)[1] |
| 1 g/L | |
| Hazards | |
| GHS pictograms | 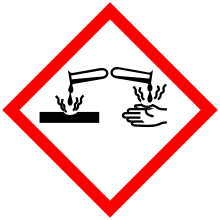  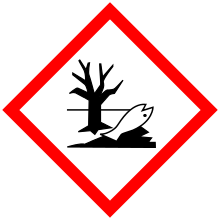 |
| GHS Signal word | Danger |
GHS hazard statements |
H302, H315, H317, H318, H400 |
| P261, P264, P270, P272, P273, P280, P301+312, P302+352, P305+351+338, P310, P321, P330, P332+313, P333+313, P362, P363, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Usage
Benzisothiazolinone has a microbicide and a fungicide mode of action. It is widely used as a preservative, for example in:
- emulsion paints, caulks, varnishes, adhesives, inks, and photographic processing solutions
- home cleaning and car care products; laundry detergents, stain removers and fabric softeners;
- industrial settings, for example in textile spin-finish solutions, leather processing solutions, preservation of fresh animal hides and skins
- agriculture in pesticide formulations
- gas and oil drilling in muds and packer fluids preservation.[2]:iv
In paints, it is commonly used alone or as a mixture with methylisothiazolinone. Typical concentrations in products are 200–400 ppm depending on the application area and the combination with other biocides. According to a study in Switzerland, 19% of the paints, varnishes and coatings contained BIT in 2000. The fraction in adhesives, sealants, plasters and fillers was shown at that time as 25%.[3] A later study in 2014 shows a dramatic rise in usage, to 95.8% of house paints.[4]
Health hazards
Given sufficient dose and duration, dermal exposure can produce skin sensitization and allergic contact dermatitis,[5] and is classified as an irritant for skin and eyes.[1] Benzisothiazolinone has also been linked with Systemic Contact Dermatitis via airborne contact.[6]
In 2012, the Scientific Committee on Consumer Safety in Europe found BIT's "sensitising potential is of concern...Sensitisation from related isothiazolinones is an important problem in consumers. This has occurred because there has been consumer exposure before safe levels of exposure relevant to sensitisation have been established. Benzisothiazolinone is a skin sensitiser in animal models with potency similar to methylisothiazolinone. Methylisothiazolinone, at 100 ppm (0.01%) in cosmetic products is causing contact allergy and allergic contact dermatitis in the consumer. Benzisothiazolinone is known to be a sensitiser in man and has induced sensitisation at circa 20 ppm in gloves."[7]
The opinion further states: "There is no information on what may be safe levels of exposure to benzisothiazolinone in cosmetic products from the point of view of sensitisation. Until safe levels of exposure have been established, the use of benzisothiazolinone in cosmetic products as a preservative or for other functions cannot be considered safe in relation to sensitisation."
Later, in 2013, researchers published a study that set out to derive the highest concentration of BIT in certain consumer products that would result in exposures below the No Expected Sensitization Induction Level (NESIL); that is, where normal use would yield a dose below the level at which skin sensitization might occur. The products under consideration were sunscreen, laundry detergent, dish soap, and spray cleaner; by way of calculation they derived BIT NESILs of 0.0075%, 0.035%, 0.035%, 0.021%, respectively. They then performed a pilot examination via bulk sample analysis of one representative product from each category labelled as containing BIT. Their findings showed all BIT concentrations well below the derived NESIL, with 0.0009% and 0.0027% for sunscreen and dish soap, respectively, and no detection in the laundry detergent and spray cleaner products, meaning the concentration was at or below the limit of detection of 0.0006%.[8]
References
- Record of 1,2-Benzisothiazol-3(2H)-one in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 22 October 2007.
- US EPA. September 29, 2005 EPA Reregistration Decision
- Reinhard; et al. (2001). "Preservation of products with MCI/MI in Switzerland". Contact Dermatitis. 45 (5): 257–64. doi:10.1034/j.1600-0536.2001.450501.x. PMID 11722483.
- Schwensen JF, Lundov MD, Bossi R, Banerjee P, Giménez-Arnau E, Lepoittevin JP, Lidén C, Uter W, Yazar K, White IR, Johansen JD (2015). "Methylisothiazolinone and benzisothiazolinone are widely used in paint: a multicentre study of paints from five European countries". Contact Dermatitis. 72 (3): 127–38. doi:10.1111/cod.12322. PMID 25510184.CS1 maint: multiple names: authors list (link)
- ToxNet 1,2-Benzisothiazoline-3-one
- Diljit Kaur-Knudsen, Torkil Menné, Berit Christina Carlsen (2012). "Systemic allergic dermatitis following airborne exposure to 1,2-benzisothiazolin-3-one". Contact Dermatitis. 67 (5): 310–312. doi:10.1111/j.1600-0536.2012.02117.x. PMID 23039006.CS1 maint: multiple names: authors list (link)
- Scientific Committee on Consumer Safety (SCCS) Opinion on Benzisothiazolinone
- Novick RM, Nelson ML, Unice KM, Keenan JJ, Paustenbach DJ (2013). "Estimation of the safe use concentrations of the preservative 1,2-benzisothiazolin-3-one (BIT) in consumer cleaning products and sunscreens". Food Chem Toxicol. 56: 60–6. doi:10.1016/j.fct.2013.02.006. PMID 23429043.CS1 maint: multiple names: authors list (link)
Literature
- Wilfried Paulus: Directory of Microbicides for the Protection of Materials and Processes. Springer Netherland, Berlin 2006, ISBN 1-4020-4861-0.
External links
