Avian malaria
Avian malaria is a parasitic disease of birds, caused by parasite species belonging to the genera Plasmodium and Hemoproteus (phylum Apicomplexa, class Haemosporidia, family Plasmoiidae).[1] The disease is transmitted by a dipteran vector including mosquitoes in the case of Plasmodium parasites and biting midges for Hemoproteus. The range of symptoms and effects of the parasite on its bird hosts is very wide, from asymptomatic cases to drastic population declines due to the disease, as is the case of the Hawaiian honeycreepers.[2] The diversity of parasites is large, as it is estimated that there are approximately as many parasites as there are species of hosts. Co-speciation and host switching events have contributed to the broad range of hosts that these parasites can infect, causing avian malaria to be a widespread global disease, found everywhere except Antarctica.
| Avian malaria | |
|---|---|
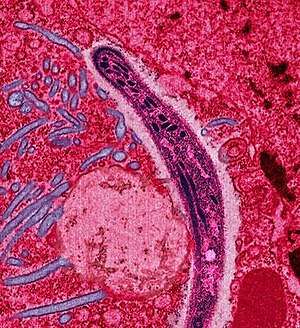 | |
| a plasmodium causing malaria | |
| Scientific classification | |
| Domain: | Eukaryota |
| (unranked): | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | P. relictum and others of the genus |
Cause
Avian malaria is most notably caused by Plasmodium relictum, a protist that infects birds in all parts of the world apart from Antarctica. There are several other species of Plasmodium that infect birds, such as Plasmodium anasum and Plasmodium gallinaceum, but these are of less importance except, in occasional cases, for the poultry industry. The disease is found worldwide, with important exceptions.[3] Usually, it does not kill birds. However, in areas where avian malaria is newly introduced, such as the islands of Hawaiʻi, it can be devastating to birds that have lost evolutionary resistance over time.
Parasite species
Avian malaria is a vector-transmitted disease caused by protozoa in the genera Plasmodium and Haemoproteus; these parasites reproduce asexually within bird hosts and both asexually and sexually within their insect vectors, which include mosquitoes (Culicidae), biting midges (Ceratopogonidae), and louse flies (Hippoboscidae).[4] The blood-parasites of the genus Plasmodium and Haemoproteus, encompass an extremely diverse group of pathogens with global distribution.[5] The large number of parasite lineages along with their wide range of potential host species and the pathogen's capacity for host switching makes the study of this system extremely complex.[1] Evolutionary relationships between hosts and the parasites have only added complexity and suggested extensive sampling is needed to elucidate how global cospeciation events drive disease transmission and maintenance in various ecosystems.[6] In addition to this, the parasite's ability to disperse can be mediated by migratory birds and thus increases variation in prevalence patterns and alters host-parasite adaptation processes.[7] Host susceptibility is highly variable as well and numerous efforts have been made to understand the relationship between increased prevalence and host traits such as nesting and foraging height, sexual dimorphism or even incubation time length. So far, the effects of this disease in wild populations is poorly understood.
There exists much controversy on what corresponds as a species in avian malaria parasites. The Latin binomials nomenclature used to describe Plasmodium and Hemoproteus parasites is based on a restricted set of morphological characteristics and the restriction to which parasites of birds they are able to infect.[6] Therefore, considering co-speciation events or even species diversity for malaria parasites is surrounded by much disagreement. Molecular tools have directed classification towards a phylogenetic definition of lineages, based on sequence divergence and the range of hosts in which the parasite can be found. The diversity of avian malaria parasites and other haemosporidia is extremely large, and previous studies have found that the number of parasites approximates the number of hosts, with significant host switching events and parasite sharing.[1] The current approach suggests amplification of the cytochrome b gene of the parasite and the reconstruction of genealogies based on this information. Due to the large amount of lineages and different host species, a public database called MalAvi has been created to encourage sharing these sequences and aid in understanding the diversity of these parasites.[8] Considering that no other genetic markers have been developed for this group of parasites, a ~1.2-4% sequence divergence has been determined as a cutoff value to distinguish between different parasite lineages.[6] The molecular approach has also allowed direct comparisons between host phylogenies and parasite genealogies, and significant co-speciation has been found based on event-based-matching of phylogenetic trees.
Phylogeny of malaria parasites
To date, there is no specific phylogeny for avian malaria parasites and related haemosporidian parasites. However, given that malaria parasites can be found in reptiles, birds and mammals, it is possible to combine the data from these groups and a well resolved large phylogeny is available.[9] For over a century, parasitologists classified malaria parasites based on morphological and life-history traits and new molecular data shows that these have variable phylogenetic signals. The current approach suggests that Plasmodium species infecting birds and squamate reptiles belong to one clade, and mammalian lineages belonging to a separate clade. In the case of Haemoproteus, this group has traditionally been classified based on the vector host, with one clade being transmitted to columbiform birds by hippoboscid flies and a second group transmitted by biting midges to other avian families. The molecular data supports this approach and suggests reclassifying the later group as Parahaemoproteous.
Phylogeography of avian malaria
Although a widespread disease, the culprit most commonly associated with the disease is Plasmodium relictum and associated lineages. To better understand the parasite's epidemiology and geographical distribution, analysis of genetic variation across large geographical scales have been conducted by looking at the nuclear gene MSP1 (merozoite surface protein) from Plasmodium relictum [10]. Findings have revealed that there are significant differences between lineages from the New and Old World, suggesting different introductions of the parasite to avian populations. In addition to this, considerable variation was found between Europe and African lineages, suggesting different patterns of transmission for temperate and tropical populations. Although this approach is relatively recent, detecting allelic variation in different markers is essential to unveil parasite transmission patterns and the likelihood of introduction to new susceptible host populations.
Vector
Its real vector in Hawaiʻi is the mosquito Culex quinquefasciatus, which was introduced to the Hawaiʻian Islands in 1826. Since then, avian malaria and avipoxvirus together have devastated the native bird population, resulting in many extinctions. Hawaiʻi has more extinct birds than anywhere else in the world; just since the 1980s, ten unique birds have disappeared.
Virtually every individual of endemic species below 4,000 feet (1,200 m) in elevation has been eliminated by the disease. These mosquitoes are limited to lower elevations, below 5,000 feet (1,500 m), by cold temperatures that prevent larval development. However, they appear to be slowly gaining a foothold at higher elevations and their range may be expanding upwards.[11] If so, most remaining Hawaiian land birds may become at risk to extinction.
Most of the Hawaiian Islands have a maximum elevation of less than 5,000 feet (1,500 m), so with the exception of the island of Hawaiʻi and East Maui, native birds may become extinct on every other island if the mosquito is able to occupy higher elevations.
Disease process and epidemiology
Plasmodium relictum reproduces in red blood cells. If the parasite load is sufficiently high, the bird begins losing red blood cells, causing anemia (USDI and USGS 2005). Because red blood cells are critical for moving oxygen about the body, loss of these cells can lead to progressive weakness and, eventually, death (USDI and USGS 2005). Malaria mainly affects passerines (perching birds). In Hawaiʻi, this includes most of the native Hawaiian honeycreepers and the Hawaiian crow. Susceptibility to the disease varies between species, for example, the ʻiʻiwi is very susceptible to malaria while the ʻApapane less so (USDI and USGS 2005). Native Hawaiʻian birds are more susceptible than introduced birds to the disease and exhibit a higher mortality rate (Van Riper et al. 1982; Atkinson et al. 1995). This has serious implications for native bird faunas (SPREP) with P. relictum being blamed for the range restriction and extinctions of a number of bird species in Hawaiʻi, primarily forest birds of low-land forests habitats where the mosquito vector is most common (Warner 1968; Van Riper 1991; USDI and USGS 2005).
The incidence of this disease has nearly tripled in the last 70 years. Notable among the species of birds most heavily affected were house sparrows, great tits, and Eurasian blackcaps. Prior to 1990, when global temperatures were cooler than now, less than 10 percent of house sparrows (Passer domesticus) were infected with malaria. In recent years, however, this figure has increased to nearly 30 percent. Likewise, since 1995, the percent of malaria-infected great tits has risen from 3 percent to 15 percent. In 1999, some 4 percent of blackcaps—a species once unaffected by avian malaria—were infected. For tawny owls in the UK, the incidence had risen from two or three percent to 60%.[12]
Control
The main way to control avian malaria is to control mosquito populations. Hunting and removing pigs helps, because wallows from feral pigs and hollowed out logs of the native hapu'u ferns provide dirty standing water where the mosquito breeds (USDI and USGS 2005). Around houses, reducing the number of potential water catchment containers helps reduce the mosquito breeding sites (SPREP Undated). However, in Hawaiʻi, attempts to control the mosquitoes by larval habitat reduction and larvicide use have not eliminated the threat.
It may also be possible to find birds that are resistant to malaria, collect eggs and raise young birds for re-introduction into areas where birds are not resistant, giving the species a head-start on spreading resistance. There is evidence for evolution of resistance to avian malaria in two endemic species, Oʻahu ʻamakihi and Hawaiʻi ʻamakihi. If other species can be preserved for long enough, they may evolve resistance as well. One tactic would be to reforest high-elevation areas on the island of Hawaiʻi, for example above the refuge of Hakalau on land managed by the Department of Hawaiʻian Homelands. This could give birds more time to adapt before climate change or mosquito evolution bring avian malaria to the last remaining bird populations.
Extirpating mosquitos from Hawai'i using CRISPR editing has also been suggested.[13]
References
- Ricklefs, R. E.; Fallon, S. M. (2002-05-07). "Diversification and host switching in avian malaria parasites". Proceedings of the Royal Society of London B: Biological Sciences. 269 (1494): 885–892. doi:10.1098/rspb.2001.1940. ISSN 0962-8452. PMC 1690983. PMID 12028770.
- van Riper, Charles; van Riper, Sandra G.; Goff, M. Lee; Laird, Marshall (1986-02-01). "The Epizootiology and Ecological Significance of Malaria in Hawaiian Land Birds". Ecological Monographs. 56 (4): 327–344. doi:10.2307/1942550. ISSN 1557-7015. JSTOR 1942550.
- Clark, Nicholas; Clegg, S.; Lima, M. (2014). "A review of global diversity in avian haemosporidians (Plasmodium and Haemoproteus: Haemosporida): new insights from molecular data". International Journal for Parasitology. 44 (5): 329–338. doi:10.1016/j.ijpara.2014.01.004. hdl:10072/61114. PMID 24556563.
- Gediminas, Valkiunas (2005-01-01). Avian malarial parasites and other haemosporidia. Taylor & Francis. ISBN 9780415300971. OCLC 476614868.
- Bensch, Staffan; Pérez-Tris, Javier; Waldenström, Jonas; Hellgren, Olof; Poulin, R. (2004-07-01). "Linkage between nuclear and mitochondrial dna sequences in avian malaria parasites: multiple cases of cryptic speciation?". Evolution. 58 (7): 1617–1621. doi:10.1554/04-026. ISSN 0014-3820.
- RICKLEFS, ROBERT; FALLON, SYLVIA; BERMINGHAM, ELDREDGE (2004-02-01). "Evolutionary Relationships, Cospeciation, and Host Switching in Avian Malaria Parasites". Systematic Biology. 53 (1): 111–119. CiteSeerX 10.1.1.496.1628. doi:10.1080/10635150490264987. ISSN 1063-5157. PMID 14965906.
- Pérez-Tris, Javier; Bensch, Staffan (2005-08-01). "Dispersal increases local transmission of avian malarial parasites". Ecology Letters. 8 (8): 838–845. doi:10.1111/j.1461-0248.2005.00788.x. ISSN 1461-0248.
- Bensch, Staffan; Hellgren, Olof; Pérez-Tris, Javier (2009-09-01). "MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages". Molecular Ecology Resources. 9 (5): 1353–1358. doi:10.1111/j.1755-0998.2009.02692.x. ISSN 1755-0998. PMID 21564906.
- Martinsen, Ellen S.; Perkins, Susan L.; Schall, Jos J. (2008-04-01). "A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): Evolution of life-history traits and host switches". Molecular Phylogenetics and Evolution. 47 (1): 261–273. doi:10.1016/j.ympev.2007.11.012. PMID 18248741.
- Hellgren, Olof; Atkinson, Carter T.; Bensch, Staffan; Albayrak, Tamer; Dimitrov, Dimitar; Ewen, John G.; Kim, Kyeong Soon; Lima, Marcos R.; Martin, Lynn (2015-08-01). "Global phylogeography of the avian malaria pathogen Plasmodium relictum based on MSP1 allelic diversity". Ecography. 38 (8): 842–850. doi:10.1111/ecog.01158. ISSN 1600-0587.
- "How to Protect Hawaii's Rarest Birds From Avian Malaria". Audubon. 2015-11-16. Retrieved 2020-02-04.
- GaramszegI, László Z (2011). "Climate change increases the risk of malaria in birds". Global Change Biology. 17 (5): 1751–1759. Bibcode:2011GCBio..17.1751G. doi:10.1111/j.1365-2486.2010.02346.x.
- Specter, Michael (15 July 2016). "How the DNA Revolution Is Changing Us". National Geographic Magazine.