August Beer
August Beer (German: [beːɐ̯]; 31 July 1825 – 18 November 1863) was a German physicist, chemist, and mathematician of Jewish descent.[1]
Biography
Beer was born in Trier, where he studied mathematics and natural sciences. Beer was educated at the technical school and gymnasium of his native town until 1845, when he went to Bonn to study mathematics and the sciences under the mathematician and physicist Julius Plücker, whose assistant he became later. In 1848 he won the prize for his essay, "De Situ Axium Opticorum in Crystallis Biaxibus," and obtained the degree of Ph.D. Two years later he was appointed lecturer at the University of Bonn.
In 1852, Beer published a paper on the absorption of red light in coloured aqueous solutions of various salts.[2] Beer makes use of the fact, derived from Bouguer's[3] and Lambert's absorption laws,[4] that the intensity of light transmitted through a solution at a given wavelength decreases exponentially with the path length d and the concentration c of the solute (the solvent is considered non-absorbing). Actually, the “Absorption Coëfficient” defined by Beer in his paper is the transmittance (or transmission ratio), T = I / I0. Thus, as pointed out by Beer, the transmittance of a concentrated solution can be derived from a measurement of the transmittance of a dilute solution.
Indeed, the transmittance measured for any concentration and path length can be normalized to the corresponding transmittance for a standard concentration and path length. Beer in a number of experiments confirms this, defining a standard concentration of 10%, and a standard path length of 10 cm. The photometer, devised by Beer, is shown in the gallery below.
Beer continued to publish the results of his scientific labors, writing in 1854 Einleitung in die höhere Optik (Introduction to the Higher Optics). His findings, together with those of Johann Heinrich Lambert, make up the Beer–Lambert law. In 1855 he was appointed professor of mathematics at the University of Bonn. Beer also wrote "Einheit in der Electrostatik," published two years after his death. He died in Bonn in 1863.[5]
Beer's law
Beer's law, also called Lambert–Beer law or Beer–Lambert law, in spectroscopy, is the physical law stating that the quantity of light absorbed by a substance dissolved in a nonabsorbing solvent is directly proportional to the concentration of the substance and the path length of the light through the solution. Beer's law is commonly written in the form A = ε cl, where A is the absorbance, c is the concentration in moles per liter, l is the path length in centimeters, and ε is a constant of proportionality known as the molar extinction coefficient. The law is accurate only for dilute solutions; deviations from the law occur in concentrated solutions because of interactions between molecules of the solute, the substance dissolved in the solvent.[6]
Gallery
 Photometer devised by Beer
Photometer devised by Beer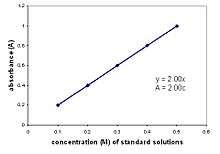 Example plot displaying the Beer–Lambert Law
Example plot displaying the Beer–Lambert Law
Selected writings
- Beer, August (1865). Einleitung in die Elektrostatik, die Lehre vom Magnetismus und die Elektrodynamik. Braunschweig: Friedrich Vieweg und Sohn.
Notes
- Charles Archibald Stonehill, The Jewish contribution to civilization, p. 23
- Beer (1852). "Bestimmung der Absorption des rothen Lichts in farbigen Flüssigkeiten" [Determination of the absorption of red light in colored liquids]. Annalen der Physik und Chemie (in German). 86 (5): 78–88.
- Bouguer, Pierre (1729). Essai d'optique sur la gradation de la lumière [Optics essay on the dimming of light] (in French). Paris, France: Claude Jombert. pp. 16–22.
- Lambert, J.H. (1760). Photometria sive de mensura et gradibus luminis, colorum et umbrae [Photometry, or, On the measure and gradations of light, colors, and shade] (in Latin). Augsburg, (Germany): Eberhardt Klett.
- Kölnische Zeitung, May 1, 1864; Poggendorff, Biographisch-Literarisches Handwörterbuch; Allgemeine Deutsche Biographie, ii. 245, 246.
- The Columbia Electronic Encyclopedia, 6th ed. Copyright © 2011, Columbia University Press.
References
- In Greenfield, E. V. (1922). Technical and scientific German. Boston: D.C. Heath & Co.
External links
- Canberra.edu.au
- Singer, Isidore; Mels, Edgar. "August Beer". Retrieved 2008-10-04.
- August Beer at the Mathematics Genealogy Project