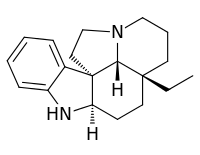
Aspidospermidine
Aspidospermidine is an alkaloid isolated from plants in the genus Aspidosperma.[1] It has been a popular target for total synthesis,[2][3][4][5] due in part to the fact that it provides a good showcase for synthetic strategies but also because the structure is similar to many other important bioactive molecules.[6]
 | |
| Names | |
|---|---|
| IUPAC name
(3aR,10bR)-3aβ-Ethyl-2,3,3a,4,5,5aα,6,11,12,13aβ-decahydro-1H-indolizino[8,1-cd]carbazole | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C19H26N2 | |
| Molar mass | 282.431 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Deutsch, Harold F.; Evenson, Merle A.; Drescher, Peter; Sparwasser, Christoph; Madsen, Paul O. (October 1994). "Isolation and biological activity of aspidospermine and quebrachamine from an Aspidosperma tree source". Journal of Pharmaceutical and Biomedical Analysis. 12 (10): 1283–1287. doi:10.1016/0731-7085(94)00066-2. PMID 7841224.
- Marino, Joseph P.; Rubio, Maria B.; Cao, Ganfeng; de Dios, Alfonso (November 2002). "Total Synthesis of (+)-Aspidospermidine: A New Strategy for the Enantiospecific Synthesis of Aspidosperma Alkaloids". Journal of the American Chemical Society. 124 (45): 13398–13399. doi:10.1021/ja026357f. PMID 12418888.
- Jones, Spencer B.; Simmons, Bryon; Mastracchio, Anthony; MacMillan, David W. C. (13 July 2011). "Collective synthesis of natural products by means of organocascade catalysis". Nature. 475 (7355): 183–188. doi:10.1038/nature10232. PMC 3439143. PMID 21753848.
- Ma, Haichen; Xie, Xingang; Jing, Peng; Zhang, Weiwei; She, Xuegong (2015). "Concise total synthesis of (±)-aspidospermidine". Org. Biomol. Chem. 13 (18): 5255–5259. doi:10.1039/C5OB00228A. PMID 25856579.
- Callaghan, Owen; Lampard, Christopher; Kennedy, Alan R.; Murphy, John A. (1999). "A novel total synthesis of (±)-aspidospermidine". Journal of the Chemical Society, Perkin Transactions 1 (8): 995–1002. doi:10.1039/A900335E.
- Anagnostaki, Elissavet E.; Zografos, Alexandros L. (2012). ""Common synthetic scaffolds" in the synthesis of structurally diverse natural products". Chemical Society Reviews. 41 (17): 5613–25. doi:10.1039/c2cs35080g. PMID 22782134.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.