Aniline (data page)
This page provides supplementary chemical data on aniline.
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source and follow its directions.
Structure and properties
| Structure and properties | |
|---|---|
| Index of refraction, nD | 1.5863 at 20 °C |
| Abbe number | ? |
| Dielectric constant,[1] εr | 6.89 ε0 at 20 °C |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
| Surface tension[2] | 44.0 dyn/cm at 10 °C 42.9 dyn/cm at 20 °C 24.4 dyn/cm at 180 °C |
| Viscosity[3] | 6.023 mPa·s at 12 °C 4.467 mPa·s at 20 °C 2.92 mPa·s at 22 °C 1.555 mPa·s at 60 °C |
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 267.13 K (–6.02 °C), ? Pa |
| Critical point | 698.8 K (425.7 °C), 4890 kPa |
| Std enthalpy change of fusion, ΔfusH |
10.54 kJ/mol |
| Std entropy change of fusion, ΔfusS |
39.57 J/(mol·K) at –6.3 °C |
| Std enthalpy change of vaporization, ΔvapH |
55.83 kJ/mol at 25 °C 42.44 kJ/mol at 184.1 °C |
| Std entropy change of vaporization, ΔvapS |
? J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
31 kJ/mol |
| Standard molar entropy, S |
191. J/(mol K) |
| Enthalpy of combustion, ΔcH |
–3393 kJ/mol |
| Heat capacity, cp | 193.7 J/(mol K) at 25 °C |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
87 kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity,[4] cp | 148.7 J/(mol K) at 25 °C |
| van der Waals' constants[5] | a = 2685 L2 kPa/mol2 b = 0.1369 liter per mole |
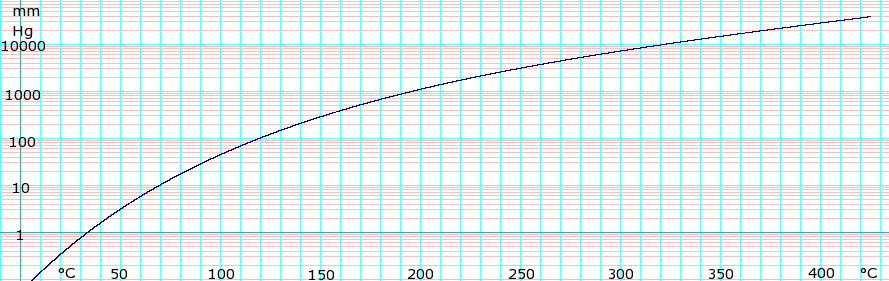
Vapor pressure of liquid
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | 1520 | 3800 | 7600 | 15200 | 30400 | 45600 | |
| T in °C | 34.8 | 69.4 | 96.7 | 119.9 | 161.9 | 184.4 | 212.8 | 254.8 | 292.7 | 342.0 | 400.0 | — | |
Table data obtained from CRC Handbook of Chemistry and Physics 44th ed.
Distillation data
See also:
- m-xylene (data page)
- p-xylene (data page)
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Spectral data
| UV-Vis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ionization potential | 7.72(62281) eV(cm−1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| λmax | 230 nm (E2-band)[7] 280 nm (B-band)[7] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Extinction coefficient, ε | 8 600 (E2-band)[7] 1 430 (B-band)[7] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Major absorption bands[8] |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Proton NMR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Carbon-13 NMR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other NMR data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Masses of main fragments |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
UV Absorbance Spectroscopy of Aniline
Aniline is a benzenoid compound. The NH2 group attached to the benzene ring means that there is a lone pair of electrons that can enter into conjugation with the benzene ring resulting in delocalization in the aniline.
Aniline absorbs in the K (220 - 250 nm) and the B (250 - 290 nm) bands exhibited by benzenoid compounds. The K and B bands arise from π to π* transitions as a result of the a group containing multiple bond being attached to the benzene ring. When dissolved in ethanol, λmax for aniline is 230 nm, but in dilute aqueous acid λmax is 203 nm. In the latter case the anilinium cation is formed and the lone pair is no longer available for conjugation with the benzene ring. Consequently, the absorption of the molecule shifts to the lower λmax value and behaves like benzene.
Regulatory data
| Regulatory data | |
|---|---|
| Flash point | 70 °C |
| RTECS | ? |
| Autoignition temperature | 615 °C |
References
- Lange's Handbook of Chemistry, 10th ed. pp 1234-1237
- Lange's Handbook of Chemistry, 10th ed. pp 1661-1663
- Lange's Handbook of Chemistry, 10th ed. pp 1669-1674
- "Pure Component Properties" (Queriable database). Chemical Engineering Research Information Center. Archived from the original on 3 June 2007. Retrieved 28 May 2007.
- Lange's Handbook of Chemistry, 10th ed, pp 1522-1524
- "Binary Vapor-Liquid Equilibrium Data" (Queriable database). Chemical Engineering Research Information Center. Retrieved 6 June 2007.
- Kaur, H. Spectroscopy. Global Media: Meerut, India, 2009; p. 304
- "Spectral Database for Organic Compounds". Advanced Industrial Science and Technology. Archived from the original (Queriable database) on 5 May 2006. Retrieved 9 June 2007.
- "NIST Standard Reference Database".
- Finar, I.L. (1974); Organic Chemistry Vol.2 - Stereochemistry and the chemistry of natural products 5th. Ed. Longman
Except where noted otherwise, data relate to standard ambient temperature and pressure.
Disclaimer applies.