Alpha amylase inhibitor
In molecular biology, alpha-amylase inhibitor is a protein family which inhibits mammalian alpha-amylases specifically, by forming a tight stoichiometric 1:1 complex with alpha-amylase. This family of inhibitors has no action on plant and microbial alpha amylases.
| A_amylase_inhib | |||||||||
|---|---|---|---|---|---|---|---|---|---|
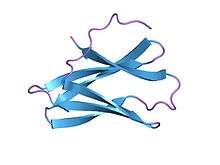 crystal structure determination, refinement and the molecular model of the alpha-amylase inhibitor hoe-467a | |||||||||
| Identifiers | |||||||||
| Symbol | A_amylase_inhib | ||||||||
| Pfam | PF01356 | ||||||||
| InterPro | IPR000833 | ||||||||
| SCOPe | 1hoe / SUPFAM | ||||||||
| |||||||||
A crystal structure has been determined for tendamistat, the 74-amino acid inhibitor produced by Streptomyces tendae that targets a wide range of mammalian alpha-amylases.[1] The binding of tendamistat to alpha-amylase leads to the steric blockage of the active site of the enzyme. The crystal structure of tendamistat revealed an immunoglobulin-like fold that could potentially adopt multiple conformations. Such molecular flexibility could enable an induced-fit type of binding that would both optimise binding and allow broad target specificity.
References
- König V, Vértesy L, Schneider TR (October 2003). "Structure of the alpha-amylase inhibitor tendamistat at 0.93 A". Acta Crystallogr. D. 59 (Pt 10): 1737–43. doi:10.1107/S0907444903015828. PMID 14501112.