Allyl bromide
Allyl bromide (3-bromopropene) is an organic halide. It is an alkylating agent used in synthesis of polymers, pharmaceuticals, and other organic compounds. Physically, allyl bromide is a colorless liquid with an intense, acrid, and persistent smell. Allyl bromide is more reactive but more expensive than allyl chloride, and these considerations guide its use.[1]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3-Bromoprop-1-ene | |
| Other names
Allyl bromide 3-Bromopropene 3-Bromopropylene 3-Bromo-1-propene Bromoallylene 2-Propenyl bromide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.134 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1099 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H5Br | |
| Molar mass | 120.977 g·mol−1 |
| Appearance | Clear to light yellow liquid |
| Density | 1.398 g/cm3 |
| Melting point | −119 °C (−182 °F; 154 K) |
| Boiling point | 71 °C (160 °F; 344 K) |
| Very slightly soluble | |
Refractive index (nD) |
1.4697 (20 °C, 589.2 nm) |
| Hazards | |
| Safety data sheet | MSDS at Oxford University |
| GHS pictograms |  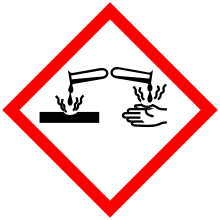   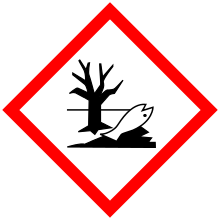 |
| GHS Signal word | Danger |
GHS hazard statements |
H225, H301, H314, H330, H331, H340, H350, H400 |
| P201, P202, P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P273, P280, P281, P284, P301+310, P301+330+331, P303+361+353, P304+340, P305+351+338, P308+313, P310, P311 | |
| NFPA 704 (fire diamond) | |
| Flash point | −2 to −1 °C |
| 280 °C (536 °F; 553 K) | |
| Explosive limits | 4.3–7.3 % |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis and reactions
It is produced commercially from allyl alcohol. Alternatively allyl chloride reacts with hydrogen bromide in the presence of copper bromide.[1]
The compound is mainly used as an electrophilic allylating agent.[2] Allylzinc bromide may be produced by treating this compound with elemental zinc.
See also
References
- Dagani, M. J.; Barda, H. J.; Benya, T. J.; Sanders, D. C. "Bromine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_405.
- José C. González-Gómez, Francisco Foubelo, Miguel Yus (2012). "Preparation of Enantioenriched Homoallylic Primary Amines". Org. Synth. 89: 88. doi:10.15227/orgsyn.089.0088.CS1 maint: multiple names: authors list (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
