Aconitic acid
Aconitic acid is an organic acid. The two isomers are cis-aconitic acid and trans-aconitic acid. The conjugate base of cis-aconitic acid, cis-aconitate is an intermediate in the isomerization of citrate to isocitrate in the citric acid cycle. It is acted upon by the enzyme aconitase.
 cis-aconitic acid | |
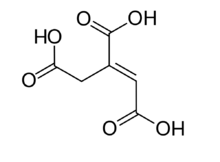 trans-aconitic acid | |
| Names | |
|---|---|
| IUPAC name
Prop-1-ene-1,2,3-tricarboxylic acid | |
| Other names
Achilleic acid; equisetic acid; citridinic acid; pyrocitric acid; achilleaic acid; acinitic acid | |
| Identifiers | |
| ChemSpider |
|
| ECHA InfoCard | 100.007.162 |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C6H6O6 | |
| Molar mass | 174.108 g·mol−1 |
| Appearance | Colorless crystals |
| Melting point | 190 °C (374 °F; 463 K) (decomposes) (mixed isomers), 173 °C (cis and trans isomers) |
| Acidity (pKa) | 2.80, 4.46 (trans isomer)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aconitic acid can be synthesized by dehydration of citric acid using sulfuric acid:[3]
- (HO2CCH2)2C(OH)CO2H → HO2CCH=C(CO2H)CH2CO2H + H2O
A mixture of isomers are generated in this way.
It was first prepared by thermal dehydration.[4]
References
- "Aconitic Acid - Compound Summary (CID 309)". PubChem.
- Dawson, R. M. C.; Elliott, D. C.; Elliott, W. H. (1989). Data for Biochemical Research (3rd ed.). Oxford: Clarendon Press. ISBN 9780198552994.
- Bruce, W. F. (1937). "Aconitic Acid". 17: 1. doi:10.15227/orgsyn.017.0001. Cite journal requires
|journal=(help) - Pawolleck, B. (1875). "Substitutionsproducte der Citronensäure und ein Versuch zur Synthese der letzteren" [Substitution products of citric acid and an attempt at the synthesis of the latter]. Justus Liebig's Annalen der Chemie. 178 (2–3): 150–170. doi:10.1002/jlac.18751780203.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.