Absorption cross section
Absorption cross section is a measure for the probability of an absorption process. More generally, the term cross section is used in physics to quantify the probability of a certain particle-particle interaction, e.g., scattering, electromagnetic absorption, etc. (Note that light in this context is described as consisting of particles, i.e., photons.) In honor of the fundamental contribution of Maria Goeppert Mayer to this area, the unit for the two-photon absorption cross section is named the "GM". One GM is 10−50 cm4 s photon−1.[1]
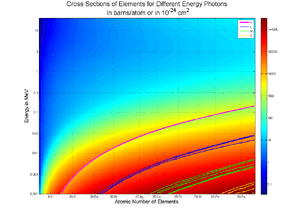
In the context of ozone shielding of ultraviolet light, absorption cross section is the ability of a molecule to absorb a photon of a particular wavelength and polarization. Analogously, in the context of nuclear engineering it refers to the probability of a particle (usually a neutron) being absorbed by a nucleus. Although the units are given as an area, it does not refer to an actual size area, at least partially because the density or state of the target molecule will affect the probability of absorption. Quantitatively, the number dN of photons absorbed, between the points x and x + dx along the path of a beam is the product of the number N of photons penetrating to depth x times the number n of absorbing molecules per unit volume times the absorption cross section σ:
- .
The absorption cross-section is closely related to molar absorptivity and mass absorption coefficient. For a given particle and its energy, the absorption cross-section of the target material can be calculated from mass absorption coefficient using:
where:
- is the mass absorption coefficient
- is the atomic molar mass in g/mol
- is Avogadro's number and is the number of molecules per mole
This is also commonly expressed as:
where:
- is the absorption coefficient
- is the atomic number density
See also
References
- "Two-Photon Absorption Measurements: Establishing Reference Standards". Australian National University. June 8, 2007. Retrieved September 14, 2013.