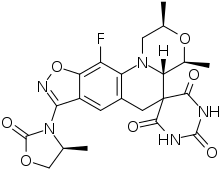
Zoliflodacin
Zoliflodacin (development codes AZD0914 and ETX0914) is an experimental antibiotic that is being studied for the treatment of infection with Neisseria gonorrhoeae (gonorrhea).[1] It has a novel mechanism of action which involves inhibition of bacterial type II topoisomerases.[2] It is being developed by Entasis Therapeutics and is (as of 2020) in Phase III clinical trials.[3]
 | |
| Clinical data | |
|---|---|
| Other names | AZD0914; ETX0914 |
| Pregnancy category |
|
| Routes of administration | Oral |
| Drug class | Antibiotic |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 97.8% |
| Metabolism | Hepatic |
| Onset of action |
|
| Elimination half-life | 5.3–6.3 h |
| Excretion | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C22H22FN5O7 |
| Molar mass | 487.444 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- Taylor SN, Marrazzo J, Batteiger BE, Hook EW, Seña AC, Long J, et al. (November 2018). "Single-Dose Zoliflodacin (ETX0914) for Treatment of Urogenital Gonorrhea". The New England Journal of Medicine. 379 (19): 1835–1845. doi:10.1056/NEJMoa1706988. PMID 30403954.
- Basarab GS, Kern GH, McNulty J, Mueller JP, Lawrence K, Vishwanathan K, et al. (July 2015). "Responding to the challenge of untreatable gonorrhea: ETX0914, a first-in-class agent with a distinct mechanism-of-action against bacterial Type II topoisomerases". Scientific Reports. 5: 11827. Bibcode:2015NatSR...511827B. doi:10.1038/srep11827. PMC 4501059. PMID 26168713.
- "Pipeline". Entasis Therapeutics.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.