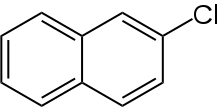
2-Chloronaphthalene
2-Chloronaphthalene is an organochlorine chemical compound, a chlorinated derivative of naphthalene. Its chemical formula is C
10H
7Cl.[1][2] The compound is an isomer for 1-chloronaphthalene.[3]
 | |
| Names | |
|---|---|
| IUPAC name
2-Chloronaphthalene | |
| Other names
β-Chloronaphthalene, 2-Chloro-naphthalene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.001.891 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H7Cl | |
| Molar mass | 162.62 g·mol−1 |
| Appearance | Off-white crystalline powder |
| Density | 1.2±0.1 g/cm3 |
| Melting point | 59 °C (138 °F; 332 K) |
| Boiling point | 255 °C (491 °F; 528 K) |
| insoluble | |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H315, H319, H335 |
| P261, P280 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
2-Chloronaphthalene is obtained directly by chlorination of naphthalene, with the formation of more highly substituted derivatives such as dichloro- and trichloronaphthalenes, in addition to the two monochlorinated isomeric compounds: 1-chloronaphthalene and 2-chloronaphthalene.[4]
Properties
2-Chloronaphthalene is a combustible, off-white odorless solid, which is practically insoluble in water. The compound may react with strong oxidizing agents.[5]
Applications
2-Chloronaphthalene can be used for the production of fullerenes.[6]
See also
References
- "2-Chloronaphthalene". EPA. comptox.epa.gov. Retrieved 14 June 2017.
- "2-Chlornaphthalin". Retrieved 14 June 2017.
- "2-Chlornaphthalin Produkt Beschreibung" (in German). chemicalbook.com. Retrieved 14 June 2017.
- Bavendamm, W.; Bellmann, H. (February 1953). "Chlornaphthalin-Präparate". Holz als Roh- und Werkstoff (in German). 11 (2): 81–84. doi:10.1007/BF02605462.
- "2-Chlornaphthalin Produkt Beschreibung" (in German). Retrieved 14 June 2017.
- Krüger, Anke (2007). Neue Kohlenstoffmaterialien: Eine Einführung (in German). Springer-Verlag. p. 53. ISBN 978-3-8351-9098-6.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.