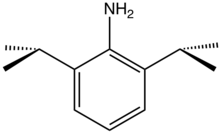
2,6-Diisopropylaniline
2,6-Diisopropylaniline is an organic compound with the formula H2NC6H3(CHMe2)2 (Me = CH3). It is a colorless liquid although, like many anilines, samples can appear yellow or brown. 2,6-Diisopropylaniline is a bulky aromatic amine that is often used to make ligands in coordination chemistry. The Schrock carbenes often are transition metal imido complexes derived from this aniline.[1] Condensation with diacetylpyridine and acetylacetone gives, respectively, diiminopyridine[2] and NacNac ligands.[3]
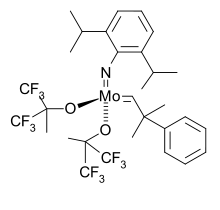
Typical Schrock-style olefin metathesis catalysts feature bulky imides as spectator ligands.
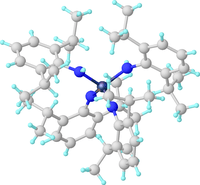
Structure of the diamido-diimido complex W(NAr)2(N(H)Ar)2 (ArNH2 = 2,6-Diisopropylaniline.[4]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,6-Di(propan-2-yl)aniline | |
| Other names
2,6-Diisopropylaniline 2,6-DIPA | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.042.081 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H19N | |
| Molar mass | 177.291 g·mol−1 |
| Appearance | colorless liquid |
| Melting point | −45 °C (−49 °F; 228 K) |
| Boiling point | 257 °C (495 °F; 530 K) |
| Hazards | |
GHS hazard statements |
H412 |
| P273, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- R. R. Schrock (2009). "Recent Advances in High Oxidation State Mo and W Imido Alkylidene Chemistry". Chemical Reviews. 109: 3211–3226. doi:10.1021/cr800502p. PMC 2726908.CS1 maint: uses authors parameter (link)
- V. C. Gibson, M. J. Humphries, K. P. Tellmann, D. F. Wass, A. J. P. White, D. J. Williams (2001). "The nature of the active species in bis(imino)pyridyl cobalt ethylene polymerisation catalysts". Chem. Commun. doi:10.1039/b107490c. 2252.CS1 maint: uses authors parameter (link)
- Mindiola, D. J.; Holland, P. L.; Warren, T. H. (2010). "Complexes of Bulky β-Diketiminate Ligands". Inorg. Synth. 35: 1–55. doi:10.1002/9780470651568.ch1.
- Tianniu Chen, K.R . Sorasaenee, Zhongzhi Wu, J. B. Diminnie, Ziling Xue (2003). "Synthesis, Characterization and X-ray Structures of New Molybdenum Bis(imide) Amide and Silyl Complexes". Inorg. Chim. Acta. 345: 113. doi:10.1016/S0020-1693(02)01271-9.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.