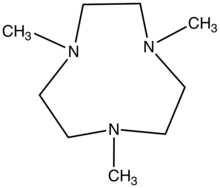
1,4,7-Trimethyl-1,4,7-triazacyclononane
1,4,7-Trimethyl-1,4,7-triazacyclononane is the heterocyclic compound with the formula (CH2CH2NCH3)3. This colorless liquid is the N-methylated derivative of triazacyclononane (TACN), a face-capping ligand that is popular in coordination chemistry. Unlike TACN, Me3TACN does not form 2:1 complexes owing to its greater bulk.[1]
 | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.119.348 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H21N3 | |
| Molar mass | 171.288 g·mol−1 |
| Appearance | Colorless oil |
| Boiling point | 207.8 °C (406.0 °F; 480.9 K) |
| Hazards | |
| GHS pictograms | 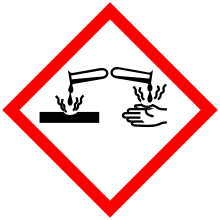  |
| GHS Signal word | Danger |
GHS hazard statements |
H314 |
| P260, P264, P270, P280, P301+312, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P330, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Several related tridentate ligands have been prepared with diverse substituents on nitrogen.[2]
ML3.png)
Generic octahedral metal complex of Me3TACN.
References
- Weighardt, Karl (1988). "1,4,7-Triazacyclononane and N,N',N"-Trimethyl-1,4,7-triazacyclononane - Two Versatile Macrocycles for the Synthesis of Monomeric and Oligomeric Metal Complexes". Pure and Applied Chemistry. 60 (4): 509–16. doi:10.1351/pac198860040509.
- Jason A. Halfen, William B. Tolman (1998). "C2 -Symmetric 1,4-Diisopropyl-7-R -1,4,7-Triazacyclononanes". C2‐Symmetric 1,4‐Diisopropyl‐7‐R‐1,4,7‐Triazacyclononanes. Inorganic Syntheses. 32. pp. 75–81. doi:10.1002/9780470132630.ch12. ISBN 9780470132630.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.