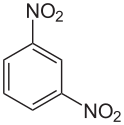
1,3-Dinitrobenzene
1,3-Dinitrobenzene is an organic compound with the formula C6H4(NO2)2. It is one of three isomers of dinitrobenzene. The compound is a yellow solid that is soluble in organic solvents.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,3-Dinitrobenzene | |
| Other names
meta-dinitrobenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.524 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1597 3443 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H4N2O4 | |
| Molar mass | 168.108 g·mol−1 |
| Appearance | yellow solid |
| Density | 1.575 g/cm3 |
| Melting point | 89.6 °C (193.3 °F; 362.8 K) |
| Boiling point | 297 °C (567 °F; 570 K) |
| Hazards | |
| GHS pictograms |   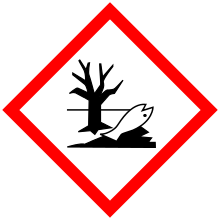 |
| GHS Signal word | Danger |
GHS hazard statements |
H300, H310, H330, H373, H400, H410 |
| P260, P262, P264, P270, P271, P273, P280, P284, P301+310, P302+350, P304+340, P310, P314, P320, P321, P322, P330, P361, P363, P391, P403+233, P405, P501 | |
| Flash point | 149°C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
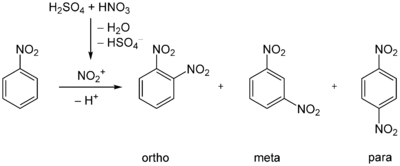
1,3-Dinitrobenzene is accessible by nitration of nitrobenzene. The reaction proceeds under acid catalysis using sulfuric acid. The directing effect of the nitro group of nitrobenzene leads to 93% of the product resulting from nitration at the meta-position. The ortho- and para-products occur in only 6% and 1%, respectively.[1]
Reactions
Reduction of 1,3-dinitrobenzene with sodium sulfide in aqueous solution leads to 3-nitroaniline. Further reduction with iron and hydrochloric acid (HCl) gives m-phenylenediamine.[2]
References
- Joachim Buddrus (2003). Grundlagen der organischen Chemie (3 ed.). Berlin: de Gruyter. p. 360. ISBN 3-11-014683-5.
- Hans Beyer and Wolfgang Walter (1981). Lehrbuch der Organischen Chemie (19 ed.). Stuttgart: S. Hirzel Verlag. pp. 536, 542. ISBN 3-7776-0356-2.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.