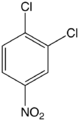
1,2-Dichloro-4-nitrobenzene
1,2-Dichloro-4-nitrobenzene is an organic compound with the formula 1,2-Cl2C6H3-4-NO2. This pale yellow solid is related to 1,2-dichlorobenzene by the replacement of one H atom with a nitro functional group. This compound is an intermediate in the synthesis of agrochemicals.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,2-Dichloro-4-nitrobenzene | |
| Other names
DCNB, 3,4-dichloronitrobenzene | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.513 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2811 1578 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H3Cl2NO2 | |
| Molar mass | 192.01 |
| Appearance | colourless to yellow needles |
| Density | 1.4588 g/cm3 |
| Melting point | 52.8 to 56 °C (127.0 to 132.8 °F; 325.9 to 329.1 K) |
| Boiling point | 263 °C (505 °F; 536 K) |
| organic solvents | |
| Hazards | |
| GHS pictograms |    |
| GHS Signal word | Danger |
GHS hazard statements |
H302, H336, H361, H372, H373, H401, H411 |
| P201, P202, P260, P261, P264, P270, P271, P273, P281, P301+312, P304+340, P308+313, P312, P314, P330, P391, P403+233, P405, P501 | |
| Flash point | 124 °C (255 °F; 397 K) |
| 420 °C (788 °F; 693 K) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production and uses
The nitration of 1,2-dichlorobenzene mainly produces 1,2-dichloro-4-nitrobenzene, together with smaller amounts of the 3-nitro isomer. It can also be prepared by chlorination of 1-chloro-4-nitrobenzene.[1]
One of the chlorides is reactive toward nucleophiles. Potassium fluoride gives 2-chloro-1-fluoro-4-nitrobenzene, an intermediate in the production of herbicides. With ammonia, one obtains 2-chloro-4-nitroaniline, a precursor to diazo dyes. Reduction with iron powder gives 3,4-dichloroaniline (m.p. 72 °C, CAS# 95-76-1).[1]
References
- Gerald Booth (2007). "Nitro Compounds, Aromatic" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, Weinheim, 2005.