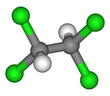
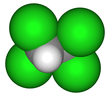
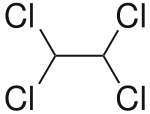
1,1,2,2-Tetrachloroethane
1,1,2,2-Tetrachloroethane is a chlorinated derivative of ethane. It has the highest solvent power of any chlorinated hydrocarbon.[1] As a refrigerant, it is used under the name R-130.
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,1,2,2-Tetrachloroethane | |||
| Other names
s-Tetrachloroethane Acetylene tetrachloride R-130 TeCA | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.089 | ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H2Cl4 | |||
| Molar mass | 167.848 g/mol | ||
| Appearance | Colorless to pale yellow liquid[2] | ||
| Odor | pungent, chloroform-like[2] | ||
| Density | 1.59 g/cm3 | ||
| Melting point | −44 °C (−47 °F; 229 K) | ||
| Boiling point | 146.5 °C (295.7 °F; 419.6 K) | ||
| 1 g/350 mL | |||
| Vapor pressure | 5 mmHg (20°C)[2] | ||
| -89.8·10−6 cm3/mol | |||
| Hazards | |||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration) |
1000 ppm (rat, 4 hr)[3] | ||
LCLo (lowest published) |
1000 ppm (rat, 4 hr) 643 ppm (mouse, 2 hr) 2714 ppm (cat, 45 min)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 5 ppm (35 mg/m3) [skin][2] | ||
REL (Recommended) |
Ca TWA 1 ppm (7 mg/m3) [skin][2] | ||
IDLH (Immediate danger) |
Ca [100 ppm][2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
It was once widely used as a solvent and as an intermediate in the industrial production of trichloroethylene, tetrachloroethylene, and 1,2-dichloroethylene.[4] However, 1,1,2,2-tetrachloroethane is no longer used much in the United States due to concerns about its toxicity.[5]
Chronic inhalation exposure in humans results in jaundice and an enlarged liver, headaches, tremors, dizziness, numbness, and drowsiness. The U.S. Environmental Protection Agency has classified it as a Group C possible human carcinogen.[5]
For occupational exposure limits, the Occupational Safety and Health Administration has set a permissible exposure limit for dermal exposures at 5 ppm over an eight-hour time-weighted average. The National Institute for Occupational Safety and Health has a more protective recommended exposure limit for dermal exposures at 1 ppm over an eight-hour time-weighted average.[6]
See also
References
- Merck Index, 11th Edition, 9125.
- NIOSH Pocket Guide to Chemical Hazards. "#0598". National Institute for Occupational Safety and Health (NIOSH).
- "1,1,2,2-Tetrachloroethane". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for 1,1,2,2-Tetrachloroethane (Update). U.S. Public Health Service, U.S. Department of Health and Human Services, Atlanta, GA. 1996.
- Tetrachloroethane at U.S. EPA
- CDC - NIOSH Pocket Guide to Chemical Hazards
5.westron is a non-inflammable liquid and is used as solvent