Vilsmeier–Haack reaction
| Vilsmeier–Haack reaction | |
|---|---|
| Named after | Anton Vilsmeier Albrecht Haack |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | vilsmeier-reaction |
| RSC ontology ID | RXNO:0000055 |
The Vilsmeier–Haack reaction (also called the Vilsmeier reaction) is the chemical reaction of a substituted amide (1) with phosphorus oxychloride and an electron-rich arene (3) to produce an aryl aldehyde or ketone (5). The reaction is named after Anton Vilsmeier and Albrecht Haack.[1][2][3] The reaction of a substituted amide with phosphorus oxychloride gives a substituted chloroiminium ion (2), also called the Vilsmeier reagent. The initial product is an iminium ion (4b), which is hydrolyzed to the corresponding aromatic ketone or aldehyde during workup.
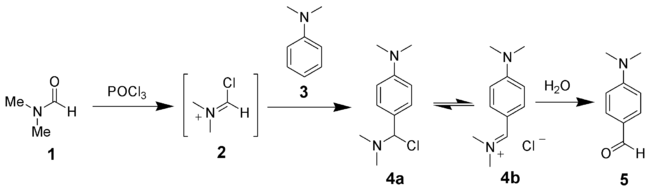 The Vilsmeier–Haack reaction
The Vilsmeier–Haack reaction
For example, benzanilide and dimethylaniline react with phosphorus oxychloride to produce an unsymmetrical diaryl ketone.[4] Similarly, anthracene can be formylated exclusively at the 9-position.[5] The reaction of anthracene with N-methylformanilide, also using phosphorus oxychloride, is shown below:
 N-Methylformanilide and anthracene and phosphorus oxychloride
N-Methylformanilide and anthracene and phosphorus oxychloride
Reaction mechanism
The reaction of the amide with phosphorus oxychloride produces an electrophilic iminium cation. The subsequent electrophilic aromatic substitution produces an iminium ion intermediate, which is hydrolyzed to give the desired aryl ketone or aryl aldehyde.[6]
 Vilsmeier–Haack reaction mechanism
Vilsmeier–Haack reaction mechanism
Modifications
In 2005, Bélanger et al. developed a new Vilsmeier–Haack type cyclization to generate the enaminals of various ring size, with endo- and exo-nitrogen.[7] Diverse tethered nonaromatic π-nucleophiles, such as silyl enol ethers, allylsilanes and enamines, could be chemoselectively added to activated amides. In contrast to the classical reaction conditions employing phosphorus oxychloride, the iminium intermediate was generated using triflic anhydride.
Applications
One recent application of this reaction involved a new synthetic route to tris(4-formylphenyl)amine[8] from triphenylamine which by known procedures resulted in a poor chemical yield of 16%. It was found that this low yield was caused by deactivation of the remaining benzene ring by the imine groups on the other two phenyl groups in the third formylation step. The procedure was modified by taking the reaction to a diimine compound followed by hydrolysis to the di-formyl compound and then (with final position reactivated) a separate formylation to the trisubstituted compound.
 Top: low-yield known procedure
Top: low-yield known procedure
Bottom: modified procedure
See also
References
- ↑ Vilsmeier, Anton; Haack, Albrecht (1927). "Über die Einwirkung von Halogenphosphor auf Alkyl-formanilide. Eine neue Methode zur Darstellung sekundärer und tertiärer p-Alkylamino-benzaldehyde" [On the reaction of phosphorus halides with alkyl formanilides. A new method for the preparation of secondary and tertiary p-alkylaminobenzaldehydes]. Berichte der Deutschen Chemischen Gesellschaft zu Berlin. 60: 119–122. doi:10.1002/cber.19270600118.
- ↑ Meth-Cohn, O.; Stanforth, S. P. (1991). "The Vilsmeier–Haack Reaction (Review)". Compr. Org. Synth. 2: 777–794. doi:10.1016/B978-0-08-052349-1.00049-4.
- ↑ Campaigne, E.; Archer, W. L. "Formylation of dimethylaniline". Organic Syntheses. 33: 27. doi:10.15227/orgsyn.033.0027. ; Collective Volume, 4, p. 331
- ↑ Hurd, C. D.; Webb, C. N. "Vilsmeyer–Haack reaction of benzanilide and dimethylaniline". Organic Syntheses. 7: 24. doi:10.15227/orgsyn.007.0024. ; Collective Volume, 1, p. 217
- ↑ Fieser, F. L.; Hartwell, J. L.; Jones, J. E.; Wood, J. H.; Bost, R. W. "Formylation of anthracene". Organic Syntheses. 20: 11. doi:10.15227/orgsyn.020.0011. ; Collective Volume, 3, p. 98
- ↑ Jones, G.; Stanforth, S. P. (2000). "The Vilsmeier Reaction of Non-Aromatic Compounds". Org. React. 56 (2): 355–686. doi:10.1002/0471264180.or056.02.
- ↑ Bélanger, G.; Larouche-Gauthier, R.; Ménard, F.; Nantel, M.; Barabé, F. (2005). "Addition of Tethered Nonaromatic Carbon Nucleophiles to Chemoselectively Activated Amides". Org. Lett. 7 (20): 4431. doi:10.1021/ol0516519.
- ↑ Mallegol, T.; Gmouh, S.; Aït Amer Meziane, M.; Blanchard-Desce, M.; Mongin, O. (2005). "Practical and Efficient Synthesis of Tris(4-formylphenyl)amine, a Key Building Block in Materials Chemistry". Synthesis. 2005: 1771–1774. doi:10.1055/s-2005-865336.