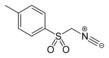
TosMIC
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1-(Isocyanomethylsulfonyl)-4-methylbenzene | |||
| Other names
Toluenesulfonylmethyl isocyanide | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.048.293 | ||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| C9H9NO2S | |||
| Molar mass | 195.24 g·mol−1 | ||
| Melting point | 109 to 113 °C (228 to 235 °F; 382 to 386 K) | ||
| Hazards | |||
| R-phrases (outdated) | R23/24/25 | ||
| S-phrases (outdated) | S36/37 S45 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
TosMIC (toluenesulfonylmethyl isocyanide) is an organic compound with the formula CH3C6H4SO2CH2NC. The molecule contains both sulfonyl and isocyanide groups. It is a colourless solid that unlike many isocyanides, is odorless. It is prepared by dehydration of the related formamide derivative. It is used in the Van Leusen reaction which is used to convert aldehydes to nitriles or in the preparation of oxazoles[2] and imidazoles.[3] The versatility of TosMIC in organic synthesis has been documented.[4] It is a fairly strong carbon acid, with an estimated pKa of 14 (compared to 29 for methyl tolyl sulfone), the isocyano group acting as an electron acceptor of strength comparable to an ester group.[5]
References
- ↑ p-Toluenesulfonylmethyl isocyanide at Sigma-Aldrich
- ↑ Keeri, Abdul Raheem; Gualandi, Andrea; Mazzanti, Andrea; Lewinski, Janusz; Cozzi, Pier Giorgio (2015-12-21). "Me2Zn-Mediated Catalytic Enantio- and Diastereoselective Addition of TosMIC to Ketones". Chemistry – A European Journal. 21 (52): 18949–18952. doi:10.1002/chem.201504362. ISSN 1521-3765.
- ↑ Hoogenboom, B. E.; Oldenziel, O. H.; van Leusen, A. M. (1977). "p-TOLYLSULFONYLMETHYL ISOCYANIDE". Organic Syntheses. 57: 102. ; Collective Volume, 6, p. 987
- ↑ "Toluenesulphonylmethyl isocyanide (TOSMIC) and the van Leusen MCR". www.organic-chemistry.org. Retrieved 2017-02-28.
- ↑ van Leusen, Albert M.; van Leusen, Daan; Czakó, Barbara (2008-09-15), "p-Tolylsulfonylmethyl Isocyanide", Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Ltd, doi:10.1002/047084289x.rt150.pub2, ISBN 0471936235, retrieved 2018-07-09
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.