C2-Symmetric ligands
In homogeneous catalysis, a C2-symmetric ligands usually describes bidentate ligands that are dyssymmetric but not asymmetric by virtue of their C2-symmetry. Such ligands have proven valuable in catalysis.[1] With C2 symmetry, C2-symmetric ligands limit the number of possible reaction pathways and thereby increase enantioselectivity, at least relative to asymmetrical analogues. Most chiral ligands combine with metals to form chiral catalyst engages in a chemical reaction in which chirality is transfer to the reaction product.
Examples
The first such ligand, the diphosphine DiPAMP, was developed in 1968 by William S. Knowles and coworkers of Monsanto Company, who shared the 2001 Nobel Prize in Chemistry.[2] This ligand was used in the industrial production of L-DOPA.
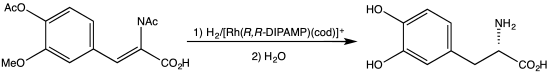 Synthesis of L-DOPA via hydrogenation with C2-symmetric diphosphine.
Synthesis of L-DOPA via hydrogenation with C2-symmetric diphosphine.
Certain classes of [C2-Symmetric ligands are called privileged ligands,[3][4]
- Ligands and Complexes
-DIOP.svg.png)
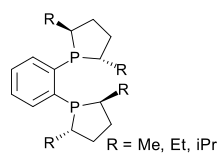 DuPhos ligands are a class of C2-symmetric ligands for asymmetric hydrogenation.[6]
DuPhos ligands are a class of C2-symmetric ligands for asymmetric hydrogenation.[6]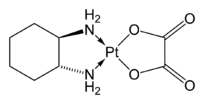 Oxaliplatin, containing the C2-symmetric R,R-diaminocyclohexane ligand, is an important anticancer drug.
Oxaliplatin, containing the C2-symmetric R,R-diaminocyclohexane ligand, is an important anticancer drug..png) Jacobsen's epoxidation catalyst is a complex of a C2-symmetric salen-type ligand.
Jacobsen's epoxidation catalyst is a complex of a C2-symmetric salen-type ligand.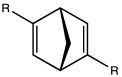 C2-symmetric diene ligand.[7]
C2-symmetric diene ligand.[7]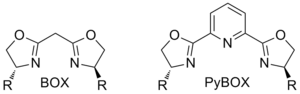 Both bi- and tridentate bis(oxazoline) ligands are used in organic synthesis
Both bi- and tridentate bis(oxazoline) ligands are used in organic synthesis Both enantiomers of BINAP
Both enantiomers of BINAP BINOL, another binaphthalene-based ligand
BINOL, another binaphthalene-based ligand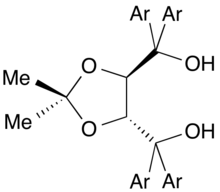
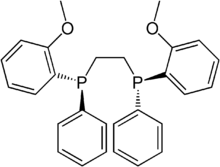 DIPAMP, a diphosphine of historic significance
DIPAMP, a diphosphine of historic significance2PHAL.png)
Mechanistic concepts
While the presence of any symmetry element within a ligand intended for asymmetric induction might appear counterintuitive, asymmetric induction only requires that the ligand be chiral (i.e. have no improper rotation axis). Asymmetric (i.e. absence of any symmetry elements) is not required. C2‑symmetry improves the enantioselectivity of the complex by reducing the number of transition states with a unique geometry. Steric/kinetic factors then usually favour the formation of a single product.[1][8]
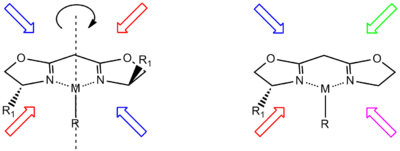
Chiral fence
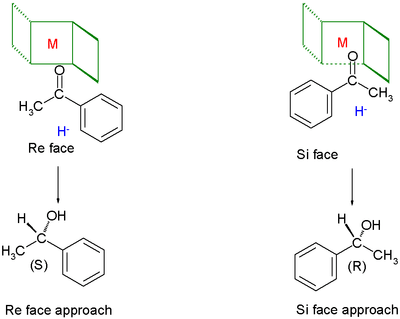
Chiral ligands work asymmetric induction somewhere along the reaction coordinate. The image depicted on the right gives a general idea how a chiral ligand may induce an enantioselective reaction. The ligand (in green) has C2 symmetry with its nitrogen, oxygen or phosphorus atoms hugging a central metal atom (in red). In this particular ligand the right side is sticking out and its left side points away. The substrate in this reduction is acetophenone and the reagent (in blue) a hydride ion. In absence of the metal and the ligand the re face approach of the hydride ion gives the (S)-enantiomer and the si face approach the (R)-enantiomer in equal amounts (a racemic mixture like expected). The ligand/metal presence changes all that. The carbonyl group will coordinate with the metal and due to the steric bulk of the phenyl group it will only be able to do so with its si face exposed to the hydride ion with in the ideal situation exclusive formation of the (R) enantiomer. The re face will simply hit the chiral fence.[9] Note that when the ligand is replaced by its mirror image the other enantiomer will form and that a racemic mixture of ligand will once again yield a racemic product. Also note that if the steric bulk of both carbonyl substituents is very similar the strategy will fail.
Other C2-symmetric complexes
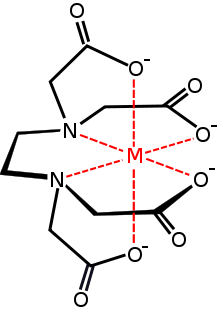
Many C2-symmetric complexes are known. Some arise, not from C2-symmetric ligands, but from the orientation or disposition of high symmetry ligands within the coordination sphere of the metal. Notably EDTA and triethylenetetraamine form complexes that are C2-symmetric by virtue of the way the ligands wrap around the metal centers. Two isomers are possible for (indenyl)2MX2, Cs- and C2-symmetric. The C2-symmetric complexes are optically stable.
Asymmetric ligands
Asymmetric ligands remain important ligands in catalysis. Examples include cinchona alkaloids and certain phosphoramidites. P-Chiral monophosphines have also been investigated.
See also
Further reading
- Desimoni, G.; Faita, G.; Jorgensen, K. A. (2006). "C2-Symmetric Chiral Bis(Oxazoline) Ligands in Asymmetric Catalysis Chem. Rev". 106: 3561–3651. doi:10.1021/cr0505324.
- Liu, X.; Lin, L.; Feng, X. (2011). "Chiral N,N'-Dioxides: New Ligands and Organocatalysts for Catalytic Asymmetric Reactions". Acc. Chem. Res. 44pages=574-587. doi:10.1021/ar200015s.
- Evans, D. A.; Kozlowski, M. C.; Murry, J. A.; Burgey, C. S.; Campos, K. R.; Connell, B. T.; Staples, R. J. (1999). "C2-Symmetric Copper(II) Complexes as Chiral Lewis Acids. Scope and Mechanism of Catalytic Enantioselective Aldol Additions of Enolsilanes to (Benzyloxy)Acetaldehyde". J. Am. Chem. Soc. 121, 669-685. doi:10.1021/JA9829822.
- Gao, J.-X.; Ikariya, T.; Noyori, R. (1996). "A Ruthenium(II) Complex with a C2-Symmetric Diphosphine/Diamine Tetradentate Ligand for Asymmetric Transfer Hydrogenation of Aromatic Ketones". Organometallics. 15: 1087–1089. doi:10.1021/OM950833B.
- Pye, P. J.; Rossen, K.; Reamer, R. A.; Tsou, N. N.; Volante, R. P.; Reider, P. J. (1997). "New Planar Chiral Bisphosphine Ligand for Asymmetric Catalysis: Highly Enantioselective Hydrogenations under Mild Conditions". J. Am. Chem. Soc. 119: 6207–6208. doi:10.1021/JA970654G.
References
- 1 2 James K. Whitesell (1989). "C2 Symmetry and Symmetric Induction". Chem. Rev. 89: 1581–1590. doi:10.1021/cr00097a012.
- ↑ Nobel prize 2001 www.nobelprize.org Link Archived 2007-07-13 at the Wayback Machine.
- ↑ Design of chiral ligands for asymmetric catalysis: From C2-symmetric P,P- and N,N-ligands to sterically and electronically nonsymmetrical P,N-ligands Andreas Pfaltz and William J. Drury III PNAS, April 20, 2004 vol. 101 no. 16 5723-5726 doi:10.1073/pnas.0307152101
- ↑ Privileged Chiral Catalysts Tehshik P. Yoon, Eric N. Jacobsen Science 14 March 2003: Vol. 299. no. 5613, pp. 1691 - 1693 doi:10.1126/science.1083622 PMID 12637734
- ↑ Dang, T. P.; Kagan, H. B. (1971). "The asymmetric synthesis of hydratropic acid and amino-acids by homogeneous catalytic hydrogenation". Journal of the Chemical Society D: Chemical Communications (10): 481. doi:10.1039/C29710000481.
- ↑ Burk, M. J.; Feaster, J. E.; Nugent, W. A.; Harlow, R. L. (1993). "Preparation and Use of C2-Symmetric Bis(Phospholanes): Production of a-Amino Acid Derivatives Via Highly Enantioselective Hydrogenation Reactions". J. Am. Chem. Soc. 115: 10125–10138. doi:10.1021/ja00075a031.
- ↑ Hayashi, T.; Ueyama, K.; Tokunaga, N.; Yoshida, K. (2003). "A Chiral Chelating Diene as a New Type of Chiral Ligand for Transition Metal Catalysts: Its Preparation and Use for the Rhodium-Catalyzed Asymmetric 1,4-Addition". J. Am. Chem. Soc. 125: 11508–11509. doi:10.1021/ja037367z.
- ↑ Rasappan, Ramesh; Laventine, Dominic; Reiser, Oliver (2008). "Metal-bis(oxazoline) complexes: From coordination chemistry to asymmetric catalysis". Coordination Chemistry Reviews. 252 (5–7): 702–714. doi:10.1016/j.ccr.2007.11.007.
- ↑ Chiral and C2-symmetrical bis(oxazolinylpyridine)rhodium(III) complexes: effective catalysts for asymmetric hydrosilylation of ketones Hisao Nishiyama, Hisao Sakaguchi, Takashi Nakamura, Mihoko Horihata, Manabu Kondo, and Kenji Itoh Organometallics; 1989; 8(3) pp 846 - 848; doi: 10.1021/om00105a047
- ↑ Atwood, J. L.; Hunter, W. E.; Hrncir, D. C.; Samuel, E.; Alt, H.; Rausch, M. D. (1975). "Molecular Structures of the Bis(η5-Indenyl)Dimethyl Derivatives of Titanium, Zirconium, and Hafnium". Inorganic Chemistry. 14: 1757–1762. doi:10.1021/ic50150a003.