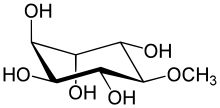
Pinitol
 | |
| Names | |
|---|---|
| IUPAC name
(1S,2S,4S,5R)-6-methoxycyclohexane-1,2,3,4,5-pentol | |
| Other names
3-O-Methyl-D-chiro-inositol D-(+)-chiro-Inositol D-Pinitol Inzitol D-(+)-Pinitol (+)-Pinitol Sennitol Pinnitol (+/-)pinitol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C7H14O6 | |
| Molar mass | 194.18 g/mol |
| Melting point | 179 to 185 °C (354 to 365 °F; 452 to 458 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pinitol is a cyclitol, a cyclic polyol. It is a known anti-diabetic agent isolated from Sutherlandia frutescens leaves.[1][2] Gall plant tannins can be differentiated by their content of pinitol.[3] It was first identified in the sugar pine (Pinus lambertiana).[4] It is also found in other plants, such as in the pods of the carob tree.[5]
Certain variants of the bacteria Pseudomonas putida have been used in organic synthesis, the first example being the oxidation of benzene, employed by Steven Ley in the synthesis of (+/-)pinitol.[6]
Glycosides
Ciceritol is a pinitol digalactoside that can be isolated from seeds of chickpea, lentil and white lupin.[7]
A cyclitol derivative can be found in the marine sponge Petrosia sp.[8]
References
- ↑ Narayanan, 1987
- ↑ Introduction Sutherlandia frutescens - Kankerbossie
- ↑ Sanz, M. L.; Martínez-Castro, I.; Moreno-Arribas, M. V. (2008). "Identification of the origin of commercial enological tannins by the analysis of monosaccharides and polyalcohols". Food Chemistry. 111 (3): 778. doi:10.1016/j.foodchem.2008.04.050.
- ↑ Anderson, A. B.; MacDonald, D. L.; Fischer, H. O. L. (1952). "The Structure of Pinitol". Journal of the American Chemical Society. 74 (6): 1479. doi:10.1021/ja01126a036.
- ↑ Tetik, N., & Yüksel, E. (2014). Ultrasound-assisted extraction of d-pinitol from carob pods using response surface methodology. Ultrasonics sonochemistry, 21(2), 860-865.
- ↑ Microbial oxidation in synthesis: A six step preparation of (+/-)-pinitol from benzene, S. V. Ley et al., Tetrahedron Lett. Volume 28, 1987, Pages 225 doi:10.1016/S0040-4039(00)95692-2
- ↑ Ciceritol, a pinitol digalactoside from seeds of chickpea, lentil and white lupin; Phytochemistry, Volume 22, Issue 8, 1983, Pages 1745-1751
- ↑ A cyclitol derivative as a replication inhibitor from the marine sponge Petrosia sp. Kim D.-K.; Young Ja Lim; Jung Sun Kim; Jong Hee Park; Nam Deuk Kim; Kwang Sik Im; Jongki Hong; Jung J. H. Journal of natural products, 1999, vol. 62, no5, pp. 773-776
External links
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.