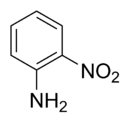
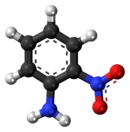
2-Nitroaniline
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Nitroaniline | |||
| Systematic IUPAC name
2-Nitrobenzenamine | |||
| Other names
ortho-Nitroaniline o-Nitroaniline | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.687 | ||
| |||
| |||
| Properties | |||
| C6H6N2O2 | |||
| Molar mass | 138.13 g·mol−1 | ||
| Appearance | Orange solid | ||
| Density | 1.442 g/mol | ||
| Melting point | 71.5 °C (160.7 °F; 344.6 K) | ||
| 0.117 g/100 ml (20°C) (SIDS) | |||
| -66.47·10−6 cm3/mol | |||
| Related compounds | |||
Related compounds |
3-Nitroaniline, 4-Nitroaniline | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
2-Nitroaniline is an organic chemical compound that is chemically described as an aniline carrying a nitro functional group in position 2. It is also classified as an aromatic amine. At ambient temperature and pressure, 2-nitroaniline is an orange solid.[1]
Synthesis
One method of preparing o-nitroaniline is via acetanilide. First, aniline acetylated with acetic anhydride.
- C6H5NH2 + (CH3CO)2O → C6H5NHC(O)CH3 + CH3CO2H
In the next step, the acetanilide is nitrated:
- C6H5NHC(O)CH3 + HNO3 → O2NC6H4NHC(O)CH3 + H2O
Finally, the nitroacetanilide is hydrolyzed:
- O2NC6H4NHC(O)CH3 + H2O → O2NC6H4NH2 + CH3CO2H
Uses
2-Nitroaniline is the main precursor to phenylenediamines, which are converted to benzimidazoles, a family of heterocycles that are key components in pharmaceuticals.[2]
Reactions
One of the factors contributing to the reactivity of 2-nitroaniline is the relative positioning of the nitro and amine groups and their activating/deactivating properties. The nitrogroup is an electron withdrawing group deactivating ortho and para positions. This leaves meta position with electron density and more likely to undergo substitution. Reinforcing this reactivity, the amino group activates ortho and para with respect to the amine. The net effect is that these sites, which are also meta to the nitro group undergo reactions.
See also
References
- ↑ Safety data for o-nitroaniline
- ↑ Gerald Booth (2007). "Nitro Compounds, Aromatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_411.