Malonate
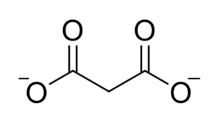
Chemical structure of the malonate ion.
The malonate or propanedioate ion is CH
2(COO)2−
2 (malonic acid minus two hydrogen ions). Malonate compounds include salts and esters of malonic acid, such as
- diethyl malonate, (C2H5)2(C3H2O4),
- dimethyl malonate, (CH3)2(C3H2O4),
- disodium malonate, Na2(C3H2O4).
Malonate is a competitive inhibitor of the enzyme succinate dehydrogenase: malonate binds to the active site of the enzyme without reacting, and so competes with succinate, the usual substrate of the enzyme. The observation that malonate is a competitive inhibitor of succinate dehydrogenase was used to deduce the structure of the active site in that enzyme.The chemical malonate decreases cellular respiration. It resembles the substrate succinate, without a −CH2CH2− group required for dehydrogenation. [1][2]
See also
References
- ↑ Potter, V. R.; Dubois, K. P. (1943). "Studies on the Mechanism of Hydrogen Transport in Animal Tissues : VI. Inhibitor Studies with Succinic Dehydrogenase". The Journal of General Physiology. 26 (4): 391–404. doi:10.1085/jgp.26.4.391. PMC 2142566. PMID 19873352.
- ↑ Dervartanian DV, Veeger C. (November 1964). "Studies on succinate dehydrogenase. I. Spectral properties of the purified enzyme and formation of enzyme-competitive inhibitor complexes". Biochim. Biophys. Acta. 92: 233–47. doi:10.1016/0926-6569(64)90182-8. PMID 14249115.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.