Macrocycle
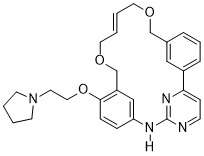
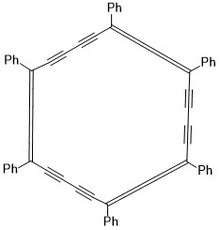
Macrocycles are often described as a molecule containing twelve or more atoms with at least one large ring.[1] The following describes the challenges for synthesis of macrocycles and two of their applications. More information can be found by looking at specific families of macrocycles linked below.
Note 1: A cyclic macromolecule has no end-groups but may nevertheless be regarded as a chain.
Note 2: In the literature, the term macrocycle is sometimes used for molecules of low relative molecular mass that would not be considered macromolecules.[2]Synthesis
The synthesis of macrocycles can be challenging for many chemists. There is not a set system in place for synthesis, many are unique to the desired molecule in question. Some require two or fewer steps[3][4] to form more complicated structures. Some utilize metals for ring closing metastases and can function as ligands for various metals.[5] The Rahman et al.'s review provides several different examples of macrocycle synthesis for several different families of macrocycles.[6] Largely the synthesis will depend on the starting material and the desired product.
Anion binding
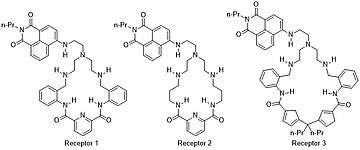
Anion transport is vital for all living organisms as it regulates ion build up in human cells. There are many different synthetic molecules chemists synthesize and utilize in order to bind, identify, and transport anions, one being macrocycles. There are two major designs for macrocycles for anion binding: cyclic amides and cyclic ureas and thioureas. Both designs rely on dipole-dipole interactions and hydrogen bonding to affectively bind various anions in differing solvents. This review provides several examples of macrocycles that have been synthesized for anion binding.[7] A specific example is Evgeny Kataev et al.'s work with three new synthesized macrocycles, pictured in Figure 2. Their macrocycles include several amine and amide functional groups with favorable pH to act as hydrogen bond donors without deprotonating the macrocycle. They tested the binding with fluoride anion and saw a turn on response for Receptor 1, Figure 2, and found up to three fluoride anions can bind.[8]
Applications in medical chemistry
Historically, there were two sources of macrocycles for drug delivery, natural products and peptides, however, more synthetic macrocycles have been developed and studied in recent years.
Macrocycles are preferred for drug delivery for the following reasons:
- Their strict conformations limit the products produced and the ability to react so there are reduced side effects.
- Macrocycles have reduced flexibility.
3 Macrocycles have reduced polarity which increases the cell permeability of the resulting molecule.[1] More polar side chains result in more active transport across a cell membrane will more nonpolar side chains passively permeate the cell.
- Intramolecular hydrogen bonds are also credited with a modest increase in cell permeability.[9]
These characteristics make macrocycles ideal as enzyme inhibitors, G protein-coupled receptors (GPCRs) and protein-protein interaction inhibitors. Due to the vast information and ways to characterize enzymes, chemists have been able to design macrocycles specifically for a specific enzyme by utilizing X-ray structures, molecular modeling, and NMR experiments. Unfortunately, this is not the case for GPCRs. Scientist often have to screen large libraries to determine what will and won't work. Macrocycles are largely used as protein-protein interaction inhibitors because their restricted confirmations make them ideal mimics.[1]
Subdivisions
See also
References
- 1 2 3 Marsault, Eric; Peterson, Mark L. (2011-04-14). "Macrocycles Are Great Cycles: Applications, Opportunities, and Challenges of Synthetic Macrocycles in Drug Discovery". Journal of Medicinal Chemistry. 54 (7): 1961–2004. doi:10.1021/jm1012374. ISSN 0022-2623.
- ↑ R. G. Jones, J. Kahovec, R. Stepto, E. S. Wilks, M. Hess, T. Kitayama, W. V. Metanomski (2008). IUPAC. Compendium of Polymer Terminology and Nomenclature, IUPAC Recommendations 2008 (the “Purple Book”) (PDF). RSC Publishing, Cambridge, UK.
- ↑ Abdelraheem, Eman M. M.; Khaksar, Samad; Kurpiewska, Katarzyna; Kalinowska-Tłuścik, Justyna; Shaabani, Shabnam; Dömling, Alexander (2018-02-02). "Two-Step Macrocycle Synthesis by Classical Ugi Reaction". The Journal of Organic Chemistry. 83 (3): 1441–1447. doi:10.1021/acs.joc.7b02984. ISSN 0022-3263.
- ↑ Zhao, Meng-Yao; Wang, De-Xian; Wang, Mei-Xiang (2018-02-02). "Synthesis, Structure, and Properties of Corona[6]arenes and Their Assembly with Anions in the Crystalline State". The Journal of Organic Chemistry. 83 (3): 1502–1509. doi:10.1021/acs.joc.7b03136. ISSN 0022-3263.
- ↑ Van Raden, Jeff M.; Louie, Shayan; Zakharov, Lev N.; Jasti, Ramesh (2017-03-01). "2,2′-Bipyridyl-Embedded Cycloparaphenylenes as a General Strategy To Investigate Nanohoop-Based Coordination Complexes". Journal of the American Chemical Society. 139 (8): 2936–2939. doi:10.1021/jacs.7b00359. ISSN 0002-7863.
- ↑ Iyoda, Masahiko; Yamakawa, Jun; Rahman, M. Jalilur (2011-11-04). "Conjugated Macrocycles: Concepts and Applications". Angewandte Chemie International Edition. 50 (45): 10522–10553. doi:10.1002/anie.201006198. ISSN 1521-3773.
- ↑ Choi, Kihang; Hamilton, Andrew D. "Macrocyclic anion receptors based on directed hydrogen bonding interactions". Coordination Chemistry Reviews. 240 (1–2): 101–110. doi:10.1016/s0010-8545(02)00305-3.
- ↑ Oshchepkov, Aleksandr S.; Shumilova, Tatiana A.; Namashivaya, Siva R.; Fedorova, Olga A.; Dorovatovskii, Pavel V.; Khrustalev, Viktor N.; Kataev, Evgeny A. (2018-02-16). "Hybrid Macrocycles for Selective Binding and Sensing of Fluoride in Aqueous Solution". The Journal of Organic Chemistry. 83 (4): 2145–2153. doi:10.1021/acs.joc.7b03077. ISSN 0022-3263.
- ↑ Ermert, Philipp (2017-10-25). "Design, Properties and Recent Application of Macrocycles in Medicinal Chemistry". CHIMIA International Journal for Chemistry. 71 (10): 678–702. doi:10.2533/chimia.2017.678.
Further reading
- Chambron, J-C.; Dietrich-Buchecker, C.; Hemmert, C.; Khemiss, A-K.; Mitchell, D.; Sauvage, J-P.; Weiss, J. (1990). "Interlacing molecular threads on transition metals" (PDF). Pure Appl. Chem. 62 (6): 1027–34. doi:10.1351/pac199062061027.