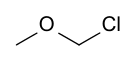
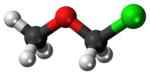
Chloromethyl methyl ether
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Chloro(methoxy)methane | |
| Other names
MOM-Cl, CMME, MCD, Chlorodimethyl ether, Chloromethoxymethane, Dimethylchloroether, Methylchloromethyl ether | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.165 |
| EC Number | 203-480-1 |
| KEGG | |
PubChem CID |
|
| RTECS number | KN6650000 |
| UNII | |
| UN number | 1239 |
| |
| |
| Properties | |
| C2H5ClO | |
| Molar mass | 80.51 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Irritating and acrid |
| Density | 1.06 g/mL |
| Melting point | −103.5 °C (−154.3 °F; 169.7 K) |
| Boiling point | 55–57 °C (131–135 °F; 328–330 K) |
| reacts | |
| Solubility | Soluble in alcohol and diethylether |
| Vapor pressure | 192 mmHg (21°C)[2] |
| Hazards | |
| Main hazards | Carcinogen & Irritant |
| Safety data sheet | https://www.cdc.gov/niosh/docs/81-123/pdfs/0129.pdf |
| GHS pictograms |    |
| GHS signal word | Danger |
| H225, H302, H312, H319, H332, H350 | |
| P201, P202, P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P281, <abbr class="abbr" title="Error in hazard statements">P301+P312, P302+352, P303+361+353, P304+312, P304+340, P305+351+338, P308+313, P312, P322, P330, P337+313 | |
| NFPA 704 | |
| Flash point | 0 °C (32 °F; 273 K) (open cup)[2] |
| US health exposure limits (NIOSH): | |
PEL (Permissible) |
OSHA-Regulated Carcinogen, no PEL[2] |
REL (Recommended) |
Carcinogenic[2] |
IDLH (Immediate danger) |
N.D.[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chloromethyl methyl ether (CMME) is a compound with formula CH3OCH2Cl. It is a chloroalkyl ether. It is used as an alkylating agent and industrial solvent to manufacture the detergent dodecylbenzyl chloride, water repellents and ion-exchange resins. In organic synthesis, it is used for introducing the methoxymethyl (MOM) protecting group,[3] and is thus often called MOM-Cl or MOM chloride.
A convenient method to prepare MOM chloride in situ is by using dimethoxymethane and an acyl chloride in the presence of catalytic Lewis acid.[4] A very similar method, using a high-boiling acyl chloride, can be used to prepare pure material. This method yields > 93% pure material with dimethoxymethane as the only contaminant.[5] In contrast, the venerable procedure from formaldehyde, methanol and hydrogen chloride[6] yields material contaminated with a significant amount of the dangerous bis(chloromethyl) ether and requires fractional distillation.
CMME is a known human carcinogen.[7] Chronic exposure can increase the incidence of respiratory cancers, including small cell carcinoma.[8] It is one of 13 chemicals regulated by the Occupational Safety and Health Administration despite not having an established permissible exposure limit.[9][10]
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[11] It listed in Schedule 1 Part 1 of Canada's Prohibition of Certain Toxic Substances Regulations.[12]
References
- ↑ Sigma-Aldrich
- 1 2 3 4 5 "NIOSH Pocket Guide to Chemical Hazards #0129". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Protective Groups in Organic Synthesis, T. W. Greene and P. G. M. Green, 3rd Edition, pages 27-33. ISBN 0-471-16019-9
- ↑ Synthesis of Alpha-Halo Ethers from Symmetric Acetals and In Situ Methoxymethylation of an Alcohol, Organic Syntheses, Vol. 84, No. 102 (2007)
- ↑ R.J. Linderman, M. Jaber, B.D. Griedel, J. Org. Chem. 1994, 59, 6499-6500.
- ↑ Monochloromethyl ether, Organic Syntheses I, 377
- ↑ bis(Chloromethyl) Ether and Technical-Grade Chloromethyl Methyl Ether CAS Nos. 542-88-1 and 107-30-2 Report on carcinogens, eleventh edition
- ↑ "Chloromethyl methyl ether". U.S. Environmental Protection Agency, Integrated Risk Information System. 7 March 2011. Accessed 3 May 2011.
- ↑ NIOSH Pocket Guide to Chemical Hazards
- ↑ "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities" (PDF) (July 1, 2008 ed.). Government Printing Office. Retrieved October 29, 2011.
- ↑ http://laws-lois.justice.gc.ca/PDF/SOR-2012-285.pdf
