Lysophosphatidylcholine
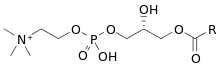
Lysophosphatidylcholines (LPC, lysoPC), also called lysolecithins, are a class of chemical compounds which are derived from phosphatidylcholines.[1]
Overview
They result from partial hydrolysis of phosphatidylcholines, which removes one of the fatty acid groups. The hydrolysis is generally the result of the enzymatic action of phospholipase A2.[2] Among other properties, they activate endothelial cells during early atherosclerosis[3][4] and stimulate phagocyte recruitment when released by apoptotic cells.[5] Moreover, LPCs can be used in the lab to cause demyelination of brain slices, to mimic the effects of demyelinating diseases such as multiple sclerosis. Further, they are known to stimulate phagocytosis of the myelin sheath and can change the surface properties of erythrocytes.[6] LPC-induced demyelination is thought to occur through the actions of recruited macrophages and microglia which phagocytose nearby myelin. Invading T cells are also thought to mediate this process. Bacteria such as Legionella pneumophila utilize phospholipase A2 end-products (fatty acids and lysophospholipids) to cause host cell (macrophage) apoptosis through cytochrome C release.
LPCs are present as minor phospholipids in the cell membrane (≤ 3%) and in the blood plasma (8–12%).[6] Since LPCs are quickly metabolized by lysophospholipase and LPC-acyltransferase, they last only shortly in vivo. By replacing the acyl-group within the LPC with an alkyl-group, alkyl-lysophospholipids (ALP) were synthesized. These LPC analogues are metabolically stable, and several such as edelfosine, miltefosine and perifosine are under research and development as drugs against cancer and other diseases.[6][7] Lysophosphatidylcholine processing has been discovered to be an essential component of normal human brain development: those born with genes that prevent adequate uptake suffer from lethal microcephaly.[8]
LPCs occur in many foods naturally. In Starch: Chemistry and Technology third edition on page 592, the authors state that "lysophosphatidylcholine makes up about 70% of the lipids in oat starch".[9]
The anti-cancer abilities of synthetic LPC variants are special since they do not target the cell DNA but insert into the plasma membrane and cause apoptosis through influencing several signal pathways. Therefore, their effects are independent of the proliferation state of the tumor cell.[10]
Other names for phosphatidylcholine in food, products, and industry
Lysolecithin is a name used prior to the 1980s that was lost and now is found as lysophosphatidylcholine. In addition to several other names lysolecithin / lysophosphatidylcholine is also called hydrolyzed lecithin or hydrolysed lecithin or enzyme-modified lecithin. It is often shortened to LPC or lysoPC. The demyelinating effects of lysolecithin / lysophosphatidylcholine occur through topical application[11] and injection.[12]
Lysophosphatidylcholine could be the lost link in the multiple sclerosis controversy between the neurologists and the vascular surgeons since lysophosphatidylcholine has a twofold inflammation effect – lysophosphatidylcholine causes lesions on the central nervous system[13] and lysophosphatidylcholine causes vasoconstriction in the venous system.[14] More is discussed below concerning possible diseases related to excessive intake of lysophosphatidylcholine. If one has sclerosis or venous occlusion then one may want to try removing all sources of lysophosphatidylcholine as seen in these foods and products.
In the Biochemistry of Foods, Peter Eck explains recombinant DNA technologies in food. Pages 543–545 explain the use of glycerophospholipid cholesterol acyltransferase and the reaction products which are lysophospholipids. He states that "the enzyme preparation is used in egg yolk and whole eggs, in processed meats, in degumming of vegetable oils, in milk products such as cheese, and in bakery products containing eggs, such as cake products". Then he lists each one and that in milk, the enzyme produces lysophospholipids from the phospholipids. He further explains that the enzyme preparation converts phospholipids to lysophospholipids in each of the above areas. Lysophosphatidylcholine is a lysophospholipid. This is significant and shows that there are unnaturally high amounts of lysophosphatidylcholine in enzyme-modified foods.[15]
Foods and products with unnaturally high quantities of lysophosphatidylcholine
Modified coconut oil has high levels of lysophosphatidylcholine;[16] to see that naturally phosphatidylcholine is 34 percent of lipids in coconut oil and lysophosphatidylcholine is only four percent. Once modified though, all that phosphatidylcholine could become hydrolyzed and be lysophosphatidylcholine – a demylenating toxin. In 2012, there was a lot said about modified coconut oil’s benefits for tooth brushing.
Here is another use for lysophosphatidylcholine – an immune activator: vaccines.[17]
Possible link between smoking and multiple sclerosis
There is an unexplained link between smoking tobacco and poor multiple sclerosis outcome.[18] One paper suggests a link between smoking and circulating lysoPC.,[19] which may have a detrimental impact on overall health.
See also
References
- ↑ Li X, Wang L, Fang P, et al. (May 2018). "Lysophospholipids induce innate immune transdifferentiation of endothelial cells, resulting in prolonged endothelial activation". The Journal of Biological Chemistry. doi:10.1074/jbc.RA118.002752. PMID 29769317.
- ↑ Phosphatidylcholine and related lipids Archived 2009-05-31 at the Wayback Machine., lipidlibrary.co.uk
- ↑ Li X, Fang P, et al. (April 2016). "Mitochondrial Reactive Oxygen Species Mediate Lysophosphatidylcholine-Induced Endothelial Cell Activation". Arteriosclerosis, Thrombosis, and Vascular Biology. 36: 1090–100. doi:10.1161/ATVBAHA.115.306964. PMC 4882253. PMID 27127201.
- ↑ Li X, Shao Y, Fang P, et al. (March 2018). "IL-35 (Interleukin-35) Suppresses Endothelial Cell Activation by Inhibiting Mitochondrial Reactive Oxygen Species-Mediated Site-Specific Acetylation of H3K14 (Histone 3 Lysine 14)". Arteriosclerosis, Thrombosis, and Vascular Biology. 38: 599–609. doi:10.1161/ATVBAHA.117.310626. PMC 5823772. PMID 29371247.
- ↑ Lauber, K; Bohn, E; Kröber, SM; Xiao, Y (2003). "Apoptotic Cells Induce Migration of Phagocytes via Caspase-3-Mediated Release of a Lipid Attraction Signal". Cell. 113 (6): 717–730. doi:10.1016/S0092-8674(03)00422-7. PMID 12809603.
- 1 2 3 Munder, PG; Modolell M; Andreesen R; Weltzien HU; Westphal O (1979). "Lysophosphatidylcholine (Lysolecithin) and its Synthetic Analogues . Immunemodulating and Other Biologic Effects". Springer Seminars in Immunopathology. 203: 187–203. doi:10.1007/bf01891668.
- ↑ Houlihan, W; Lohmeyer M; Workman P; Cheon SH (1995). "Phospholipid antitumor agents". Medicinal Research Reviews. 15 (3): 157–223. doi:10.1002/med.2610150302. PMID 7658750.
- ↑ Guemez-Gamboa, Alicia; N Nguyen; Hongbo Yan. "Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome". Nature Genetics. 47: 809–813. doi:10.1038/ng.3311. PMC 4547531. PMID 26005868.
- ↑ "Starch". google.com.
- ↑ van Blitterswijk, W; Verheij M (2008). "Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects". Current Pharmaceutical Design. 14 (21): 2061–74. doi:10.2174/138161208785294636. PMID 18691116.
- ↑ Griffin JW; et al. (1990). "Schwann cell proliferation following lysolecithin-induced demyelination". J. Neurocytol. 19: 367–84. doi:10.1007/bf01188405. PMID 2391539.
- ↑ MouseDoctor. "Multiple Sclerosis Research: Research: Promoting remyelination is not a simple task". multiple-sclerosis-research.blogspot.com.
- ↑ Pavelko KD, van Engelen BG, Rodriguez M (1998). "Acceleration in the Rate of CNS Remyelination in Lysolecithin Induced Demyelination" (PDF). The Journal of Neuroscience. 18 (7): 2498–2505. PMID 9502810.
- ↑ "Endothelial function in proteinuric renal disease". Kidney International. 56: S57–S61. doi:10.1046/j.1523-1755.1999.07115.x.
- ↑ "Biochemistry of Foods". google.com.
- ↑ "Rahman's page 12 chart" (PDF). Archived from the original (PDF) on 2014-03-28.
- ↑ "Patent US20110135684 - Use of L-alpha-lysophosphatidylcholine to obtain the differentiation of ... - Google Patents". google.com.mx.
- ↑ Julie Stachowiak, Ph.D. "Smoking and Multiple Sclerosis - Smoking Mechanisms of Action in Multiple Sclerosis". About.com Health.
- ↑ Fratta Pasini, A; Stranieri, C; Pasini, A; Vallerio, P; Mozzini, C; Solani, E; Cominacini, M; Cominacini, L; Garbin, U. "Lysophosphatidylcholine and Carotid Intima-Media Thickness in Young Smokers: A Role for Oxidized LDL-Induced Expression of PBMC Lipoprotein-Associated Phospholipase A2?". PLOS ONE. 8: e83092. doi:10.1371/journal.pone.0083092. PMC 3866188. PMID 24358251.