Lordosis behavior

Lordosis behavior, also known as mammalian lordosis (Greek lordōsis, from lordos "bent backward"[1]) or presenting, is the naturally occurring body posture for sexual receptivity to copulation present in most mammals including rodents, elephants, and felines. The primary characteristics of the behavior are a lowering of the forelimbs but with the rear limbs extended and hips raised, ventral arching of the spine and a raising, or sideward displacement, of the tail. During lordosis, the spine curves dorsoventrally so that its apex points towards the abdomen.
The lordosis reflex is hardwired in the brain, controlled by hormones in the hypothalamus,[2] triggered by touch stimuli on flanks, rump, tailbase or perineum,[3] and facilitated by vaginal stimulation[4] and by sexual pheromones.[5]
Description
Lordosis is a reflex action that causes many non-primate female mammals to adopt a body position that is often crucial to reproductive behavior. The posture moves the pelvic tilt in an anterior direction, with the posterior pelvis rising up, the bottom angling backward and the front angling downward. Lordosis aids in copulation as it elevates the hips, thereby facilitating penetration by the penis. It is commonly seen in female mammals during estrus (being "in heat"). Lordosis occurs during copulation itself and in some species, like cat, during pre-copulatory behavior.
Neurobiology
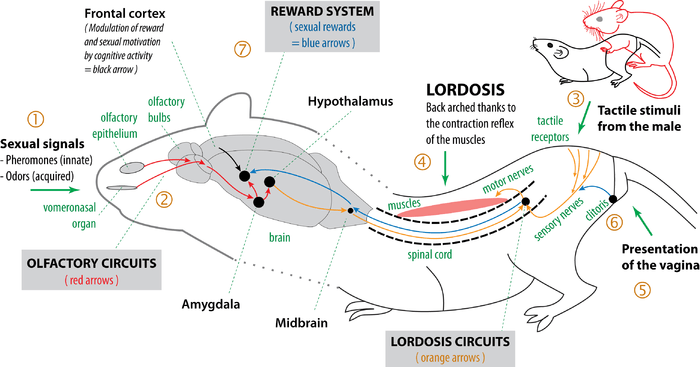
Lordosis brain circuits are connected with other innate sexual circuits, especially olfactory circuits and reward system.
In female, the main neurobiological processes of reproductive behaviour are:
- Odors, especially pheromones, make it possible to exchange sexual signals between potential partners.[9]
- Olfactory circuits (red arrows of the above diagram) make it possible to recognize the partner of the opposite sex[6][10] and to trigger sexual arousal,[7][11] which induces vaginal lubrication, erection and copulation.
- When the male mounts the female, the male's tactile stimuli on female's rump trigger the lordosis reflex.[12][3]
- The lordosis circuits (orange arrows) cause the ventral arching of the female's spine, leading to elevation of the hips and presentation of the vagina to the male, facilitating penetration by the penis.[12][3]
- The tactile contact between the penis and the female's genital area triggers the reflex movements of the male's (pelvic thrusts), followed by intromission. After intromission, thrusting movements of the penis in the vagina trigger ejaculation.[13][14]
- Tactile stimulation of the clitoris (and the penis for the male) during copulation are transmitted to the brain (blue arrows).[15]
- Activation of the reward system induces learning which optimize copulation, particularly by the development of sexual motivation.[16] Moreover, olfactive, auditive and visual signals perceived during the copulation may by conditioning become sexual signals,[17] which optimizes the innate pheromonal signals.[7]
There is thus, in the innate neurobiological organization of the organism, a true heterosexual reproductive behavior in non-primate mammals.[18][8]
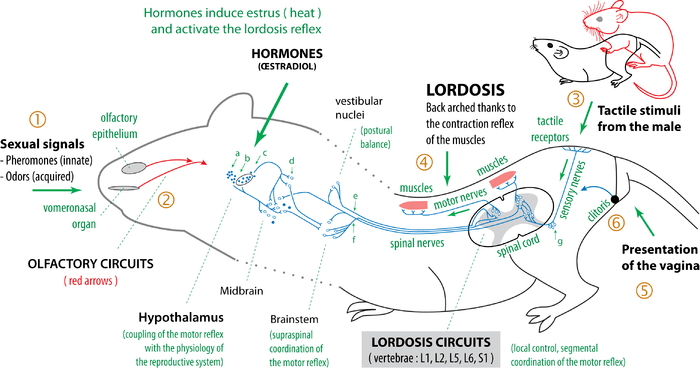

More precisely, the lordosis sexual reflex is mainly hardwired in the spinal cord, at the level of the lumbar and sacral vertebrae (L1, L2, L5, L6 and S1).[3] In the brain, several regions modulate the lordosis reflex. The vestibular nuclei and the cerebellum, via the vestibular tract, send information which makes it possible to coordinate the lordosis reflex with postural balance. More importantly, the ventromedial hypothalamus sends projections that inhibit the reflex at the spinal level. For this reason, in general, the lordosis reflex is not functional.[12] Sex hormones control reproduction and coordinate sexual activity with the physiological state. Schematically, at the breeding season, and when an ovum is available, hormones (especially estrogen) simultaneously induce ovulation and estrus (heat). Under the action of estrogen in the hypothalamus, the lordosis reflex is uninhibited.[2] The female is ready for copulation and fertilization.
During the copulation, when a male approaches the female, male pheromones (part 1 of the above diagram) are detected by the olfactory circuits (part 2). The pheromonal signals stimulate, among other things, the hypothalamus, which facilitates the lordosis reflex.[5] Then when the male mounts the female (part 3), tactile stimuli on the flanks, the perineum and the rump of the female are transmitted via the sensory nerves in the spinal cord. In the spinal cord and lower brainstem, they are integrated with the information coming from the brain, and then, in general, a nerve impulse is transmitted to the muscles via the motor nerves. The contraction of the longissimus and transverso-spinalis muscles causes the ventral arching of the vertebral column (part 4).[3] The lordosis position which results from it makes it possible to present properly the vagina to the male (part 5), facilitating penile intromission. Then, during intromission, tactile and deep sensations from the genital area and clitoris accentuate the lordosis reflex (part 6).[4] It is thus observed that the physiological and neurobiological organization of the lordosis behavior reflex is specifically adapted to heterosexual copulation.
Hormonal and cerebral regulation of lordosis
Sexual behaviour is optimized for reproduction, and the hypothalamus is the key brain area which regulates and coordinates the physiological and behavioural aspects of reproduction.[19] Most of the time, the ventromedial nucleus of the hypothalamus (VMN) inhibit lordosis. But when environmental conditions are favorable and the female is in estrus, the estrogen hormone, estradiol, induces sexual receptivity by the neurons in the ventromedial nucleus,[20] the periaqueductal gray, and other areas of the brain. The ventromedial hypothalamus sends impulses down axons synapsing with neurons in the periaqueductal gray. These convey an impulse to neurons in the medullary reticular formation which project down the reticulospinal tract and synapse with the neurobiological circuits of the lordosis reflex in the spinal cord (L1–L6). These neurobiological processes induced by estradiol enable the tactile stimuli to trigger lordosis.
The mechanisms of regulation of this estrogen-dependent lordosis reflex have been identified through different types of experiments. When the VMN is lesioned lordosis is abolished; this suggests the importance of this cerebral structure in the regulation of lordosis. Concerning hormones, displays of lordosis can be affected by avariectomy, injections of estradiol benzoate and progesterone,[21] or exposure to stress during puberty.[22][23] Specifically, stress can suppressed the hypothalamic-pituitary-gonadal (HPG) axis and therefore decrease concentrations of gonadal hormones. Consequently, these reductions in exposure to gonadal hormones around puberty can result in decreases in sexual behavior in adulthood, including displays of lordosis.[22] Lordosis can also be elicited by human manual cutaneous stimulation of the flanks followed by the rump-tail base-perineum region;[24] this allows the researcher to evaluate the effects of his experiments with a simple palpation.
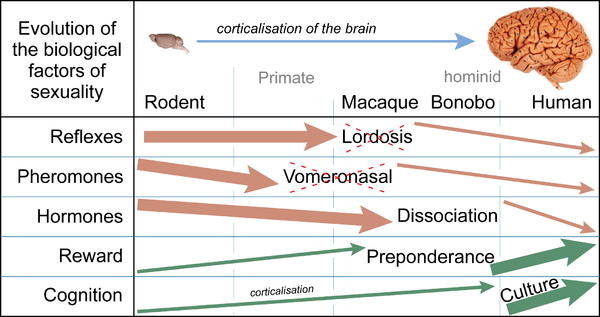
Evolution, from rodent to human
Increased corticalization of the brain induces several changes in the control of sexual behavior, including lordosis behavior.[25]
These changes induced an evolution of the stereotyped reflex sexual behaviors of non-human mammals to "the astounding variety of human sexual behaviors".[18] From the anthropoids onwards (simians/monkeys) there is a gradual loss of the lordotic reflex, in parallel with the loss of major hormone effects and estrus.[28] In the Cercopithecidae (macaques, etc.), the lordotic reflex is still functional, but it is part of sexual and sociosexual activities such as presenting. This sociosexual presenting (which follows the same sequence as lordosis: curvature of the back and lateral movement of the tail, which presents the genital region to view) is no longer dependent on hormonal control, but occurs in response to a social situation.[29] In the Hominidae, lordosis and immobilisation are absent, even during the phase around ovulation. Female receptiveness is neither obligatory nor passive. The female can actively avoid or refuse the male, or can terminate the encounter.[28]
In humans
As a result of these evolved differences, lordosis behavior becomes secondary in hominidae and is apparently non functional in humans. There is no human analogue to the lordosis reflex, although lordosis-like positions can be observed in women being mounted from behind.[30] When a woman gets onto all fours, curves her back and remains still, it is no longer a reflex movement triggered by sexual stimuli, but a voluntary movement.[25] In women, the motor reflexes involved in copulation have been replaced by new sexual activities, several of which have nothing to do with copulation nor fertilisation. Unlike lordosis, human sexual activities (kissing, mutual masturbation, fellatio, face-to-face coitus, etc.) are not motor reflexes, but are mostly voluntary and learned activities, in order to obtain sexual rewards.[18][31][32]
However, in a recent study, using 3D models and eye-tracking technology it is shown the slight thrusting out of a woman's back influence how attractive others perceive her to be and captures the gaze of both men and women.[33] The authors argue "while reflexive lordosis posture is not exhibited by human females and receptivity is not passive or obligatory for them, a manifestation of lumbar curvature might serve as a vestigial remnant of proceptivity-/receptivity-communicative signal between men and women".[34] Previously, anthropologist Helen Fisher also speculated that when a human female wears high-heeled footwear the buttocks thrust out and the back arches into a pose that simulates lordosis behavior, which is why high heels are considered "sexy".[35]
See also
References
- ↑ "lordosis". The American Heritage Dictionary. Retrieved January 3, 2017.
- 1 2 Flanagan-Cato L.M. (2011). "Sex differences in the neural circuit that mediates female sexual receptivity". Frontiers in Neuroendocrinology. 32 (2): 124–136. doi:10.1016/j.yfrne.2011.02.008. PMC 3085563. PMID 21338620.
- 1 2 3 4 5 6 7 Pfaff D. W. , Schwartz-Giblin S., Maccarthy M. M., Kow L-M (1994). "Cellular and molecular mechanisms of female reproductive behaviors", in Knobil E., Neill J. D. The physiology of reproduction, Raven Press, 2nd edition.
- 1 2 Gonzalez-Flores O., Beyer C., Lima-Hernandez F.J., Gomora-Arrati P., Gomez-Camarillo M.A., Hoffman K., Etgen A.M. (2007). "Facilitation of estrous behavior by vaginal cervical stimulation in female rats involves alpha1-adrenergic receptor activation of the nitric oxide pathway". Behavioural Brain Research. 176 (2): 237–243. doi:10.1016/j.bbr.2006.10.007. PMC 1810388. PMID 17095102.
- 1 2 Haga S., Hattori T., Sato T., Sato K., Matsuda S., Kobayakawa R., Sakano H., Yoshihara Y., Kikusui T., Touhara K. (2010). "The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor" (PDF). Nature. 466 (7302): 118–122. doi:10.1038/nature09142.
- 1 2 Stowers L., Holy T. E., Meister M., Dulac C., Koentges G. (2002). "Loss of sex discrimination and male-male aggression in mice deficient for TRP2" (PDF). Science. 295 (5559): 1493–1500. doi:10.1126/science.1069259.
- 1 2 3 Moncho-Bogani J., Lanuza E., Hernandez A., Novejarque A., Martinez-Garcia F. (2002). "Attractive properties of sexual pheromones in mice: innate or learned?". Physiology & Behavior. 77 (1): 167–176. doi:10.1016/s0031-9384(02)00842-9.
- 1 2 3 (in French) Wunsch S. (2014) To understand the origins of human sexuality. Neurosciences, ethology, anthropology. Comprendre les origines de la sexualité humaine. Neurosciences, éthologie, anthropologie. L'Esprit du Temps.
- ↑ Keller M., Bakker J. (2009). "Pheromonal communication in higher vertebrates and its implication on reproductive function. Editorial". Behavioural Brain Research. 200 (2): 237–238. doi:10.1016/j.bbr.2009.02.003.
- ↑ Dulac C., Torello A. T. (2003). "Molecular detection of pheromone signals in mammals: from genes to behaviour" (PDF). Nat. Rev. Neurosci. 4 (7): 551–562. doi:10.1038/nrn1140.
- ↑ Yoon H., Enquist L. "Olfactory inputs to hypothalamic neurons controlling reproduction and fertility". Cell. 123 (4): 669–682. doi:10.1016/j.cell.2005.08.039.
- 1 2 3 4 Kow L.M., Florea C., Schwanzel-Fukuda M., Devidze N., Kami K.H., Lee A., Zhou J., Maclaughlin D., Donahoe P., Pfaff D. (2007). "Development of a sexually differentiated behavior [lordosis] and its underlying CNS arousal functions". Curr. Top. Dev. Biol. 79: 37–59.
- ↑ Allard J., Truitt W. A., Mckenna K. E., Coolen L. M. (2005). "Spinal cord control of ejaculation". World J. Urol. 23 (2): 119–126. doi:10.1007/s00345-004-0494-9.
- ↑ Coolen L. M. (2005). "Neural control of ejaculation". J Comp Neurol. 493 (1): 39–45. doi:10.1002/cne.20784.
- ↑ Matsumoto J., Urakawa S., Hori E., de Araujo M.F., Sakuma Y., Ono T., Nishijo H. (2012). "Neuronal responses in the nucleus accumbens shell during sexual behavior in male rats". The Journal of Neuroscience. 32 (5): 1672–1686. doi:10.1523/jneurosci.5140-11.2012.
- ↑ Cibrian-Llanderal T., Tecamachaltzi-Silvaran M., Triana-Del R.R., Pfaus J.G., Manzo J., Coria-Avila G.A. (2010). "Clitoral stimulation modulates appetitive sexual behavior and facilitates reproduction in rats" (PDF). Physiology & Behavior. 100 (2): 148–153. doi:10.1016/j.physbeh.2010.02.015.
- ↑ Pfaus J.G., Kippin T.E., Coria-Avila G.A., Gelez H., Afonso V.M., Ismail N., Parada M. (2012). "Who, what, where, when (and maybe even why)? How the experience of sexual reward connects sexual desire, preference, and performance" (PDF). Archives of Sexual Behavior. 41 (1): 31–62.
- 1 2 3 Agmo A. (2007) Functional and dysfunctional sexual behavior
- ↑ Plant T., Zeleznik A. (Eds). Knobil and Neill's Physiology of Reproduction. Academic Press, 4th edition, 2015
- ↑ Kow LM, Pfaff DW (May 1998). "Mapping of neural and signal transduction pathways for lordosis in the search for estrogen actions on the central nervous system". Behav. Brain Res. 92 (2): 169–180. doi:10.1016/S0166-4328(97)00189-7. PMID 9638959.
- ↑ Olster, D.H.; Blaustein, J.D. (1989). "Development of steroid-induced lordosis in female guinea pigs: effects of different estradiol and progesterone treatments, clonidine, and early weaning". Hormones and Behavior. 23 (1): 118–129. doi:10.1016/0018-506x(89)90079-2. PMID 2538389.
- 1 2 Jasmina Kercmar; Stuart Tobet; Gregor Majdic (2014). "Social Isolation during Puberty Affects Female Sexual Behavior in Mice". Frontiers in Behavioral Neuroscience. 8: 337. doi:10.3389/fnbeh.2014.00337. PMC 4179611. PMID 25324747.
- ↑ D. Daniels; LM. Flanagan-Cato (2000). "Social Isolation during Puberty Affects Female Sexual Behavior in Mice". J Neurobiology. 45: 1–13. PMID 10992252.
- ↑ Pfaff, D.W.; Sakuma, Y. (1979). "Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus" (PDF). Journal of Physiology. 288 (1): 189–202.
- 1 2 3 Wunsch S. Phylogenesis of mammal sexuality. Analysis of the evolution of proximal factors. Sexologies 26(1):e1-e10, 2017
- ↑ Nei M., Niimura Y., Nozawa M. (2008). "The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity" (PDF). Nat. Rev. Genet. 9 (12): 951–963. doi:10.1038/nrg2480.
- ↑ Zhang J., Webb D. M. (2003). "Evolutionary deterioration of the vomeronasal pheromone transduction pathway in catarrhine primates". Proceedings of the National Academy of Sciences of the United States of America. 100 (14): 8337–8341. doi:10.1073/pnas.1331721100. PMC 166230.
- 1 2 Dixson A.F. Primate sexuality: Comparative studies of the Prosimians, Monkeys, Apes, and Human Beings. Oxford University Press, 2nd edition, 2012
- ↑ Hanby J.P. "Sociosexual development in primates." in Bateson P.P. (Ed). Perspectives in Ethology, volume 2. Plenum Press, (1):1–67, 1976
- ↑ Pfaus, J. G.; Flanagan-Cato, L. M.; Blaustein, J. D. "Female sexual behavior". in Plant T., Zeleznik A. (Eds). Knobil and Neill's Physiology of Reproduction. Academic Press, 4th edition, 2015
- ↑ Wunsch, S. (2007). Rôle et importance des processus de renforcement dans l'apprentissage du comportement de reproduction chez l'Homme. Ph.D. thesis, Sorbonne, Paris.
- ↑ Georgiadis J.R., Kringelbach M.L., Pfaus J.G. (2012). "Sex for fun: a synthesis of human and animal neurobiology" (PDF). Nat. Rev. Urol. 9 (9): 486–498. doi:10.1038/nrurol.2012.151.
- ↑ Elizabeth Hawkins (October 25, 2017). "Why arched backs are attractive". springer.com.
- ↑ Pazhoohi, F.; Doyle, J.F.; Macedo, A.F.; Arantes, J. (2017). "Arching the Back (Lumbar Curvature) as a Female Sexual Proceptivity Signal: an Eye-Tracking Study". Evolutionary Psychological Science: 1–8. doi:10.1007/s40806-017-0123-7.
- ↑ Laura T. Coffey (Sep 23, 2009). "Do high heels empower or oppress women?". TODAY msnbc.com.