Ice III
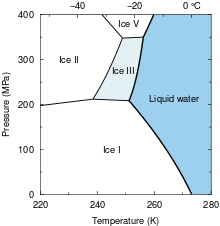
Phase diagram of water, showing the region where ice III is stable.
Ice III is a form of solid matter which consists of tetragonal crystalline ice, formed by cooling water down to 250 K at 300 MPa. It is the least dense of the high-pressure water phases, with a density of 1160 kg/m3 (at 350 MPa). The proton-ordered form of ice III is ice IX.
Ordinary water ice is known as ice Ih, (in the Bridgman nomenclature). Different types of ice, from ice II to ice XVI, have been created in the laboratory at different temperatures and pressures.
See also
- Ice, for other crystalline forms of ice
References
- Chaplin, Martin (2007-11-11). "Ice-three and ice-nine structures". Water Structure and Science. Retrieved 2008-01-02.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.