Hydroiodic acid
| |||
| |||
| Names | |||
|---|---|---|---|
| Other names
Hydronium iodide | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.030.087 | ||
| EC Number | 233-109-9 | ||
PubChem CID |
|||
| RTECS number | MW3760000 | ||
| UNII | |||
| |||
| |||
| Properties | |||
| HI(aq) | |||
| Molar mass | 127.91 | ||
| Appearance | colorless liquid | ||
| Odor | acrid | ||
| Density | 1.70 g/mL, azeotrope (57% HI by weight) | ||
| Boiling point | 127 °C (261 °F; 400 K) 1.03 bar, azeotrope | ||
| Aqueous solution | |||
| Hazards | |||
EU classification (DSD) (outdated) |
Corrosive (C) | ||
| R-phrases (outdated) | R34 | ||
| S-phrases (outdated) | (S1/2), S26, S45 | ||
| NFPA 704 | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other anions |
Hydrofluoric acid Hydrochloric acid Hydrobromic acid | ||
Related compounds |
Hydrogen iodide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Hydroiodic acid (or hydriodic acid) is a highly acidic aqueous solution of hydrogen iodide (HI) (concentrated solution usually 48 - 57% HI). It is the second strongest hydrohalic acid, after hydroastatic acid. Hydroiodic acid is a commonly used chemical reagent and is one of the strong acids that ionize completely in an aqueous solution. Concentrated hydroiodic acid has a pH of less than 0.
Reactions
Hydroiodic acid readily reacts with oxygen in air, contributing to the deep colours associated with old samples;
- 4 HI + O2 → 2 H
2O + 2 I2 - HI + I2 → HI3
Like other halogens, hydroiodic acid will perform addition reactions with unsaturated hydrocarbons such as alkenes.
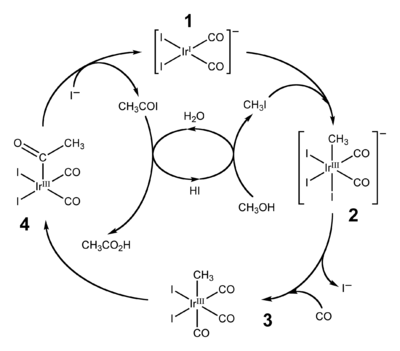
Cativa process
The Cativa process is a major end use of hydroiodic acid, which serves as a co-catalyst for the production of acetic acid by the carbonylation of methanol.[1][2]
Illicit uses
Hydroiodic acid is listed as a U.S. Federal DEA List I Chemical, owing to its use as a reducing agent related to the production methamphetamine from pseudoephedrine (recovered from nasal decongestant pills).[3] This reaction is stereospecific, producing only (d)-methamphetamine.
References
- ↑ Jones, J. H. (2000). "The CativaTM Process for the Manufacture of Acetic Acid" (PDF). Platinum Metals Rev. 44 (3): 94&ndash, 105.
- ↑ Sunley, G. J.; Watson, D. J. (2000). "High productivity methanol carbonylation catalysis using iridium - The CativaTM process for the manufacture of acetic acid". Catalysis Today. 58 (4): 293–307. doi:10.1016/S0920-5861(00)00263-7.
- ↑ Skinner, Harry F. "Methamphetamine Synthesis via HI/Red Phosphorus Reduction of Ephedrine". Forensic Science International, 48 128-134 (1990)