Azepane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Azepane | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.524 | ||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| C6H13N | |||
| Molar mass | 99.18 g·mol−1 | ||
| Density | 0.88 g/cm3[1] | ||
| Boiling point | 138 °C (280 °F; 411 K)[1] (749 mmHg) | ||
| Hazards | |||
| Flash point | 18 °C (64 °F; 291 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Azepane is a saturated heterocycle, containing one nitrogen atom in seven-membered ring.
Analogous to the well-known reaction of other amines,[2][3] azepane reacts with CO2 and in principle can be used for carbon capture.[4]
Azepane-containing drugs
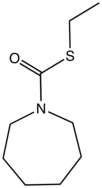
Molinate is a commercial pesticide that is derived from azepane.
- Amicibone
- Bacmecillinam (KW 1100)
- Beperidium
- Brazergoline
- Buzepide (Fenpipramide homolog).
- Cetiedil
- Gimantan
- Glidazamide
- Glisoxepide
- Glypinamide
- Hexacaine
- Mecillinam
- Molinate
- MR-16728 (cetiedil analogue)
- Nabazenil
- Pincainide
- Prozapine
- Setastine
- Tolazamide
- Pubchem 11142404
See also
References
- 1 2 "Hexamethyleneimine".
- ↑ Caplow, M. (1968). "Kinetics of Carbamate Formation and Breakdown". J. Am. Chem. Soc. 90 (24): 6795–6803. doi:10.1021/ja01026a041.
- ↑ Danckwerts, P. V. (1979). "The Reaction of CO2 with Ethanolamines". Chem. Eng. Sci. 34 (4): 443–446. doi:10.1016/0009-2509(79)85087-3.
- ↑ Sanz-Pérez, E. S.; Arencibia, A.; Sanz, R.; Calleja, G. (2016). "New developments on carbon dioxide capture using amine-impregnated silicas". Adsorption. 22 (4): 366–375. doi:10.1007/s10450-015-9740-2.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.