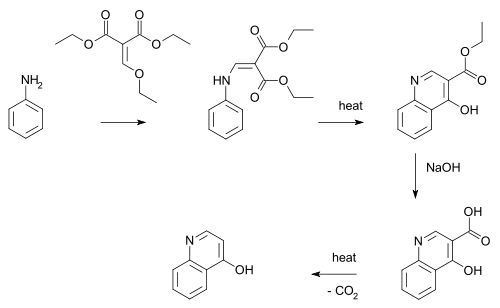
Gould–Jacobs reaction
The Gould–Jacobs reaction is an organic synthesis for the preparation of quinolines and 4‐hydroxyquinoline derivatives. The Gould-Jacobs reaction is a series of reactions. The series of reactions begins with the condensation/substitution of an aniline with alkoxy methylenemalonic ester or acyl malonic ester, producing anilidomethylenemalonic ester. Then through a 6 electron cyclization process, 4-hydroxy-3-carboalkoxyquinoline is formed, which exist mostly in the 4-oxo form. Saponification results in the formation of an acid. This step is followed by decarboxylation to give 4-hydroxyquinoline.[1] The Gould-Jacobs reaction is effective for anilines with electron‐donating groups at the meta‐position.[2]
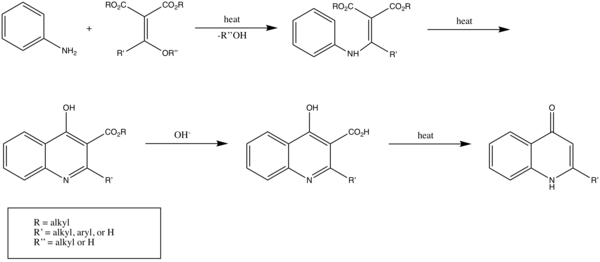
Specifically, 4-quinolinol can be synthesized.[3] In this reaction aniline or an aniline derivative first reacts with malonic acid derivative ethyl ethoxymethylenemalonate with substitution of the ethoxy group by nitrogen. A benzannulation takes place by application of heat to a quinoline. The ester group is hydrolysed by sodium hydroxide to the carboxylic acid and decarboxylation again by application of heat to 4-hydroxyquinoline.
Extension of the Gould-Jacobs approach can prepare unsubstituted parent heterocycles with fused pyridine ring of Skraup type (see Skraup reaction).[1]
Mechanism
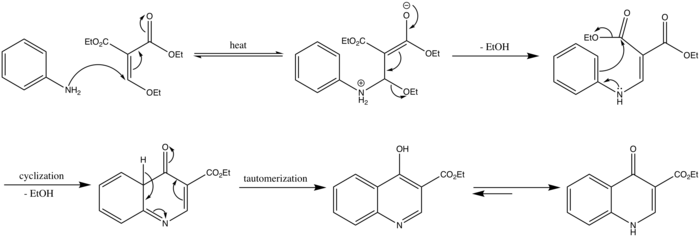
The mechanism for the Gould–Jacobs reaction begins with a nucleophilic attack from the amine nitrogen follows by the loss of ethanol to form the condensation product. A 6 electron cyclization reaction with the loss of another ethanol molecule forms a quinoline (ethyl 4-oxo-4,4a-dihydroquinoline-3-carboxylate). The enol form can be representated from the keto form through keto-enol tautomerism. Protonation of the nitrogen forms ethyl 4-oxo-1,4-dihydroquinoline-3-carboxylate.
Examples and Applications
An example is the synthesis of 4,7-dichloroquinoline [7]
- Floctafenine and Glafenine are a pair of fenamate NSAIDs whose synthesis rely on the Gould–Jacobs reaction.
- A lot of quinolone antibiotic structures such as Rosoxacin, Oxolinic acid, Droxacin, etc.
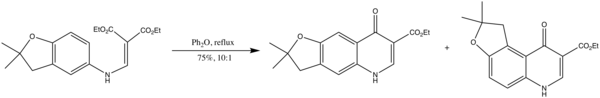
Another example is in the synthesis of antimalarials as aminoalkylamino derivatives of 2,3-dihydrofuroquinolines[8]
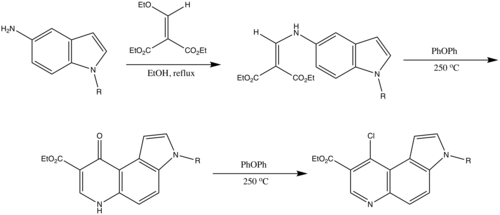
The Gould reaction is also used to convert 5-aminoindole to quinolines for the purpose of synthesizing pyrazolo[4,3-c]pyrrolo[3,2-f]quinolin-3-one derivatives as modified pyrazoloquinolinone analogs. These compounds have the potential to act as antagonists at central benzodiazepine receptors (BZRs) in Xenopus laevis oocytes.[9]
The Gould‐Jacobs reaction has also been used both conventionally with condensation steps and acyclic intermediated and with single step microwave irradiation to synthesize ethyl 4‐oxo‐8,10‐substituted‐4,8‐dihydropyrimido[1,2‐c]pyrrolo[3,2‐e]pyrimidine‐3‐carboxylates.[10]
pyrrolo(3%2C2%E2%80%90e)pyrimidine%E2%80%903%E2%80%90carboxylates_by_the_Gould-Jacobs_reaction.png)
References
- 1 2 Li, Jie Jack (2006). "Gould–Jacobs reaction". Name Reactions: A Collection of Detailed Reaction Mechanisms. Berlin, Heidelberg: Springer. pp. 289–290. ISBN 978-3-540-30030-4.
- ↑ Wang, Zerong (2010). "Gould‐Jacobs Reaction". Comprehensive Organic Name Reactions and Reagents. John Wiley & Sons, Inc. ISBN 9780471704508.
- ↑ Gould, R. Gordon; Jacobs, Walter A. (1939). "The Synthesis of Certain Substituted Quinolines and 5,6-Benzoquinolines". J. Am. Chem. Soc. 61 (10): 2890–2895. doi:10.1021/ja01265a088.
- ↑ Li, Jie Jack (2009). "Gould–Jacobs reaction". Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications (4th ed.). Springer-Verlag. pp. 263–265. doi:10.1007/978-3-642-01053-8_113. ISBN 9783642010538.
- ↑ Lengyel, László Csaba; Sipos, Gellért; Sipőcz, Tamás; Vágó, Teréz; Dormán, György; Gerencsér, János; Makara, Gergely; Darvas, Ferenc (2015). "Synthesis of Condensed Heterocycles by the Gould–Jacobs Reaction in a Novel Three-Mode Pyrolysis Reactor". Org. Process Res. Dev. 19 (3): 399–409. doi:10.1021/op500354z.
- ↑ "Gould–Jacobs Reaction". Comprehensive Organic Name Reactions and Reagents. 276: 1252–1255. 2010. doi:10.1002/9780470638859.conrr276. ISBN 9780470638859.
- ↑ Price, Charles C.; Roberts, Royston M. (1948). "4,7-Dichloroquinoline (Quinoline, 4,7-dichloro-)". Organic Syntheses. 28: 38. doi:10.15227/orgsyn.028.0038. ; Collective Volume, 3, p. 272
- ↑ Cruickshank, Philip A. (1970). "Antimalarials. 1. Aminoalkylamino derivatives of 2,3-dihydrofuroquinolines". Journal of Medicinal Chemistry. 13: 1110–1114 – via ACS Publications.
- ↑ Ferlin, Maria Grazia (2005). "Novel anellated pyrazoloquinolin-3-ones: synthesis and in vitro BZR activity". Bioorganic & Medicinal Chemistry. 13: 3531–3541 – via Elsevier Science Direct.
- ↑ Desai, Nirmal D. (2009). "The gould‐jacob type of reaction for the synthesis of novel pyrimidopyrrolopyrimidines: A comparison of classical heating vs solvent free microwave irradiation". Journal of Heterocyclic Chemistry. 43 – via Wiley Online Library.
| Wikimedia Commons has media related to Gould-Jacobs reaction. |