Glossary of chemistry terms
 |
| Chemistry |
|---|
Most of the terms listed in Wikipedia glossaries are already defined and explained within Wikipedia itself. However, glossaries like this one are useful for looking up, comparing and reviewing large numbers of terms together. You can help enhance this page by adding new terms or writing definitions for existing ones.
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment. Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it has an extensive vocabulary and a significant amount of jargon.
A
- absolute zero
- A theoretical condition concerning a system at zero kelvin, where a system does not emit or absorb energy (all atoms are at rest).
- absorbance
- abundance
- accuracy
- How close a measured value is to the actual or true value. Compare precision.
- acid
- A compound that, when dissolved in water, gives a pH of less than 7.0 or a compound that donates a hydrogen ion.
- acid anhydride
- a compound with two acyl groups bound to a single oxygen atom
- acid dissociation constant
- A quantitative measure of the strength of an acid in solution expressed as an equilibrium constant for a chemical dissociation reaction in the context of acid-base reactions. It is generally denoted by the symbol Ka. Also called acid ionization constant or acidity constant.
- actinides
- The periodic series of metallic elements with atomic numbers 89 to 103, from actinium through lawrencium. Also called actinoids.
- activated complex
- A structure that forms because of a collision between molecules while new bonds are formed.
- activation energy
- The minimum energy which must be available to a chemical system with potential reactants in order to result in a chemical reaction.
- activity series
- actual yield
- acyclic
- Containing only linear structures of atoms (particularly in hydrocarbons).
- addition reaction
- In organic chemistry, when two or more molecules combine to make a larger one.
- adhesion
- The tendency of dissimilar particles or surfaces to cling to one another as a result of intermolecular forces. Compare cohesion.
- aeration
- The mixing of air into a liquid or a solid.
- alcohol
- Any organic compound consisting of a hydroxyl functional group attached to a saturated carbon atom.
- aldehyde
- Any organic compound consisting of a carbonyl group attached to a hydrogen atom and any other R-group.
- alkali metal
- Any of the metallic elements belonging to Group 1 of the periodic table: lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr).
- alkaline earth metal
- Any of the metallic elements belonging to Group 2 of the periodic table: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
- alkane
- Any saturated acyclic hydrocarbon.
- alkene
- An unsaturated hydrocarbon containing at least one pair of double-bonded carbons.
- alkyl group
- A functional group consisting of an alkane missing a hydrogen atom.
- alkyne
- An unsaturated hydrocarbon containing at least one pair of triple-bonded carbons.
- allomer
- A substance that differs in chemical composition but has the same crystalline structure as another substance.
- allotrope
- Elements that can have different structures (and therefore different forms), such as carbon (diamonds, graphite, and fullerene).
- alloy
- A mixture of metals or of a metal and another element which in combination exhibit a metallic bonding character. Common examples include bronze, brass, and pewter.
- amalgam
- amplitude
- The maximum distance that the particles of the medium carrying the wave move away from their rest position.
- anion
- A negatively charged ion.
- anode
- 1) An electrode through which the conventional electric current (the flow of positive charges) enters into a polarized electrical circuit; 2) the wire or plate of an electrochemical cell having an excess positive charge. Negatively charged anions always move toward the anode. Compare cathode.
- aqueous solution
- A solution in which the solvent is water. It is denoted in chemical equations by appending (aq) to a chemical formula.
- aromaticity
- A chemical property of conjugated rings of atoms, such as benzene, which results in unusually high stability.
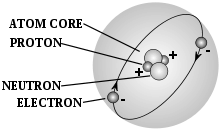
- atom
- A chemical element in its smallest form, made up of protons and neutrons within the nucleus and electrons circling the nucleus.
- atomic mass
- The mass of an atom, typically expressed in unified atomic mass units and nearly equivalent to the mass number.
- atomic mass unit
- See unified atomic mass unit.
- atomic number
- The number of protons found in the nucleus of an atom of a given chemical element. It is identical to the charge number of the nucleus and is used in the periodic table to uniquely identify each chemical element. Also called the proton number.
- atomic orbital
- the region where the electron of the atom may be found
- atomic radius
- atomic weight
- average atomic mass
- Avogadro's law
- Avogadro's number
- The number of particles in one mole of a substance (6.02×1023).
- azeotrope
- A mixture of liquids whose composition is unchanged by distillation.
B
- barometer
- A device used to measure atmospheric pressure.
- base
- A substance that accepts a proton and has a high pH; a common example is sodium hydroxide (NaOH).
- base anhydride
- Oxides of group I and II metal elements.
- beaker
- Beer–Lambert law
- biochemistry
- The study of the chemistry of biological systems and organisms.
- Bohr model
- boiling
- See vaporization.
- boiling point
- The temperature at which a liquid substance starts to boil.
- boiling-point elevation
- the process where the boiling point is elevated by adding a substance
- bond
- The attraction and repulsion between atoms and molecules that is a cornerstone of chemistry.
- Boyle's law
- for a given mass of gas at constant temperature, the volume varies inversely with the pressure
- Bragg's law
- Brønsted–Lowry acid
- Any chemical species that readily donates a proton.
- Brønsted–Lowry acid–base reaction
- Brønsted–Lowry base
- Any chemical species that readily accepts a proton.
- Büchner flask
- buckminsterfullerene
- buffered solution
- An aqueous solution consisting of a weak acid and its conjugate base or a weak base and its conjugate acid that resists changes in pH when strong acids or bases are added.
- bumping
- bung
- burette
- Glassware used to dispense specific amounts of liquid when precision is necessary (e.g. titration and resource-dependent reactions). Also called a buret.
C
- calorimeter
- A device used to measure heat.
- catalyst
- Any element or compound that facilitates an increase in the speed of a chemical reaction but which is not consumed or destroyed during the reaction. It is considered both a reactant and a product of the reaction.
- cathode
- An electrode from which the conventional electric current (the flow of positive charges) exits a polarized electrical circuit. Positively charged cations always move toward the cathode, though the cathode's polarity can be positive or negative depending on the type of electrical device and how it is being operated. Compare anode.
- cation
- A positively charged ion.
- centrifugation
- centrifuge
- equipment used to separate substances based on density by rotating the tubes around a centred axis
- cell potential
- the force in a galvanic cell that pulls electron through reducing agent to oxidizing agent
- chain reaction
- charge number
- A quantized value of electric charge calculated as the electric charge in coulombs divided by the elementary-charge constant, or z = q/e. Charge numbers for ions are denoted in superscript (e.g. Na+ indicates a sodium ion with a charge number of positive one). Atomic numbers are charge numbers of atomic nuclei.
- Charles's law
- When the pressure on a sample of a dry gas is held constant, the Kelvin temperature is directly proportional to its volume.
- chelation
- A type of bonding involving the formation of two separate coordinate covalent bonds between a polydentate ligand and a single central metal ion. The ligand is usually an organic compound called a chelant or chelating agent.
- chemical composition
- The identity and relative number of the elements that make up a chemical compound, which can often be expressed with a chemical formula.
- chemical formula
- Any of various means of concisely displaying information about the chemical composition of a compound or molecule using letters, numbers, and/or typographical symbols. Chemical formulas, such as empirical and molecular formulas, can only indicate the identities and numerical proportions of the atoms in a compound and are therefore more limited in descriptive power than chemical names and structural formulas.
- chemical law
- A law of nature relevant to chemistry, such as the law of conservation of mass.
- chemical nomenclature
- chemical process
- chemical reaction
- The change of one or more substances into another or multiple substances.
- chemical species
- chemical substance
- A form of matter that has constant chemical composition and characteristic properties and which cannot be separated into simpler components by purely physical methods (i.e. without breaking chemical bonds). It is often called a pure substance to distinguish it from a mixture.
- chemistry
- The scientific discipline that studies chemical substances, compounds, and molecules composed of atoms of various chemical elements, as well as their composition, structure, properties, behavior, and the changes they undergo during reactions with other substances.
- chirality
- cis–trans isomerism
- closed system
- cohesion
- The tendency of similar particles or surfaces to cling to one another as a result of intermolecular forces. Compare adhesion.
- colligative property
- A property of solutions that depends upon the ratio of the number of solute particles to the number of solvent particles in the solution, and not on the nature of the chemical species present.
- colloid
- A mixture of evenly dispersed substances, such as many milks.
- combustion
- An exothermic reaction between an oxidant and a fuel that produces large amounts of heat and often light.
- Commission on Isotopic Abundances and Atomic Weights (CIAAW)
- compression
- An area in a longitudinal wave where the particles are closer and pushed in.
- compound
- A substance that is made up of two or more chemically bonded elements.
- concentration
- condensation
- The phase transition of a substance from a gas to a liquid.
- condosity
- A comparative measurement of the electrical conductivity of a solution, defined as the molar concentration of a sodium chloride (NaCl) solution that has the same specific electrical conductance as the solution under test and expressed in units of moles per unit volume.
- conductor
- Any object or material that allows the flow of an electric current in one or more directions. Compare insulator.
- conformation
- The spatial arrangement of atoms affording distinction between stereoisomers which can be interconverted by rotations about formally single bonds.
- conjugate acid
- conjugate base
- conjugated system
- cooling curve
- coordinate chemistry
- coordinate covalent bond
- coordination complex
- corrosion
- An irreversible interfacial chemical reaction of a material with its environment which results in consumption of the material or dissolution into the material of an external component of the environment.
- coulomb
- The SI unit of electric charge (symbol: C), defined as the charge transported by a constant current of one ampere in one second.
- covalent bond
- A chemical bond that involves the sharing of electron pairs between atoms. The stable balance of attractive and repulsive forces that occurs between atoms when they share electrons is known as covalent bonding. A covalent bond is also called a molecular bond.
- crest
- The highest point in a wave.
- critical mass
- critical point
- crystal
- A solid whose constituent particles (such as atoms, ions, or molecules) are arranged in an orderly periodic microscopic structure, forming a lattice that extends in all directions.
- crystallography
- cuvette
- A type of glassware used in spectroscopic experiments. It is usually made of plastic, glass or quartz and should be as clean and clear as possible.
- cyclotron

An example of combustion
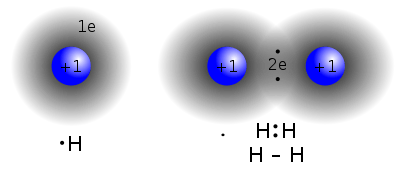
The diatomic hydrogen molecule, H2 (right), is formed by a covalent bond when two single hydrogen atoms share two electrons
D
- d-block
- Dalton's law of partial pressures
- deionization
- The removal of ions, and in water's case, mineral ions such as sodium, iron and calcium.
- deliquescence
- Substances that absorb water from the atmosphere to form liquid solutions.
- delocalized electron
- density
- An intensive property of a substance defined as mass per unit volume or d = m/V.
- denticity
- The number of donor groups in a single ligand that bind to a central atom in a coordination complex.
- dependent variable
- deposition
- The settling of particles within a solution or mixture.
- Dewar flask
- See vacuum flask.
- diatomic
- Composed of two atoms.
- diatomic molecule
- Any molecule composed of only two atoms, of the same or different elements.
- diffusion
- dimer
- dipolar bond
- dipole
- The electric or magnetic separation of charge.
- dipole moment
- The polarity of a polar covalent bond.
- dispersion
- dissociation
- dissolution
- The interaction of a solvent with the molecules or ions of a solute, involving bond formation, hydrogen bonding, and van der Waals forces. Also called solvation.
- distillation
- double bond
- A chemical bond involving the covalent sharing of two pairs of electrons.
- double-replacement reaction
- ductility
- A measure of a material's ability to undergo significant plastic deformation before rupturing, typically expressed as percent elongation or percent area reduction from a tensile test and popularly characterized by the material's ability to be stretched into a wire. Also called malleability.
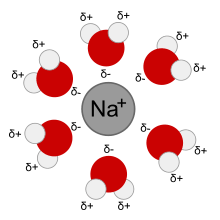
A sodium ion (Na+) forms a solvation complex with water molecules when dissolved in an aqueous solution
E
- earth metal
- See alkaline earth metal.
- electrolyte
- A solution that conducts a certain amount of electric current and can be split categorically into weak and strong electrolytes.
- electrochemical cell
- Using a chemical reaction's current, electromotive force is made.
- electromagnetic radiation
- A type of wave that can go through vacuums as well as material and is classified as a self-propagating wave.
- electromagnetic spectrum
- electromagnetism
- Fields with an electric charge and electrical properties that change the way that particles move and interact.
- electromotive force
- A device that gains energy as electric charges pass through it.
- electron
- A subatomic particle with a net charge that is negative.
- electron configuration
- electron pair
- Two electrons which occupy the same molecular orbital but have opposite spins. Electron pairs form chemical bonds or occur as lone pairs of valence electrons; it is also possible for electrons to occur as unpaired electrons.
- electron shell
- An orbital around an atom's nucleus containing a fixed number of electrons (usually two or eight).
- electronegativity
- electric charge
- A measured property (coulombs) that determines electromagnetic interaction.
- element
- A species of atoms having the same number of protons in their atomic nuclei and hence the same atomic number. Chemical elements constitute all of the ordinary matter in the universe; 118 elements have been identified and are organized by their various chemical properties in the periodic table of the elements.
- enantiomer
- enantiomorph
- endothermic process
- energy
- A system's ability to do work.
- enthalpy
- A measure of the total energy of a thermodynamic system. Usually symbolized as H.
- enthalpy of fusion
- entropy
- The amount of energy not available for work in a closed thermodynamic system. Usually symbolized as S.
- environmental chemistry
- enzyme
- A protein catalyst that speeds up a chemical reaction.
- empirical formula
- Also called the simplest formula, gives the simplest whole-number ratio of atoms of each element present in a compound.
- equilibrium
- Universally, it is the condition of a system in which all competing influences are balanced. Chemical equilibrium is the state in which the concentrations of the reactants and products have stopped changing in time.
- Eppendorf tube
- A generalized and trademarked name used for a microcentrifuge tube.
- Erlenmeyer flask
- exothermic process
- extensive property
- extraction
- extrinsic property

F
- f-block
- freezing
- The phase transition of a substance from a liquid to a solid.
- Faraday constant
- A unit of electric charge widely used in electrochemistry and equal to ~ 96,500 coulombs. It represents 1 mol of electrons, or the Avogadro number of electrons: 6.022 × 1023 electrons. F = 96 485.339 9(24) C/mol.
- Faraday's laws of electrolysis
- A two-part law that Michael Faraday published about electrolysis. The mass of a substance altered at an electrode during electrolysis is directly proportional to the quantity of electricity transferred at that electrode; the mass of an elemental material altered at an electrode is directly proportional to the element's equivalent weight.
- Fick's laws of diffusion
- filtrate
- first-order reaction
- flask
- formal charge
- fractional distillation
- free radical
- freezing-point depression
- frequency
- A measurement of the number of cycles of a given process per unit of time. The SI unit for measuring frequency is the hertz (Hz), with 1 Hz = 1 cycle per second.
- functional group
G
- galvanic cell
- A type of battery made up of electrochemicals with two different metals connected by a salt bridge.
- gas
- One of the four fundamental states of matter, characterized by high-energy particles which fill their container but have no definite shape or volume.
- gas chromatography
- Gay-Lussac's law
- A chemical law used for each of the two relationships derived by French chemist Joseph Louis Gay-Lussac and which concern the properties of gases, though the name is more usually applied to his law of combining volumes.
- geochemistry
- The study of the chemistry and chemical composition of the Earth and geological processes.
- Gibbs energy
- A value that indicates the spontaneity of a reaction. Usually symbolized as G.
- glass
- gram-atom
- One gram-atom of an element is defined as a collection of 6.023X10^23 atoms.
- Grignard reaction
- ground glass joint
- An apparatus designed to quickly and easily fit two pieces of leak-tight glassware together, featuring ground glass surfaces and typically a custom-made conical taper.
- group
- A column of the periodic table of the elements and the elements that share it. Also called a family. Compare period.
H
- halogen
- Any of the five non-metallic elements of Group 17 of the periodic table: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).
- hadron
- A subatomic particle of a type including the baryons and mesons that can take part in the strong interaction.
- heat
- Energy transferred from one system to another by thermal interaction.
- heat of fusion
- See enthalpy of fusion.
- Henry's law
- Hess's law
- Hund's rules
- hydrate
- hydration reaction
- hydrogen
- hydrogen bond
- A form of electrostatic interaction between an electronegative atom and a hydrogen atom bound to a second electronegative atom. Hydrogen bonding is unique because the small size of the hydrogen atoms permits proximity of the interacting electrical charges, and may occur as an intermolecular or intramolecular force.
- hydrogenation
- hydrolysis
- The cleavage of a chemical bond by the addition of water.
I
- ideal gas
- ideal gas constant
- The proportionality constant in the ideal gas law, defined as 0.08206 L·atm/(K·mol). Also called the universal gas constant.
- ideal gas law
- the volume of a gas is proportional to the amount of gas and its Kelvin temperature and inversely proportional to its pressure
- independent variable
- indicator
- A special compound added to a solution that changes color depending on the acidity of the solution; different indicators have different colors and effective pH ranges.
- induced radioactivity
- radioactivity caused by bombarding a stable isotope with elemental particles, forming a radioactive isotope
- inorganic compound
- Any chemical compound that does not contain carbon, though there are exceptions.
- inorganic chemistry
- A branch of chemistry concerning the chemical properties and reactions of inorganic compounds.
- insulator
- Any material that resists the flow of electric current. Compare conductor.
- intensive property
- intermediate
- intermolecular force
- International System of Units (SI)
- International Union of Pure and Applied Chemistry (IUPAC)
- An international federation of chemists that is recognized as the world authority in developing standards for chemical nomenclature and other methodologies in chemistry.
- intramolecular force
- intrinsic property
- ion
- A molecule that has gained or lost one or more electrons from its neutral state and therefore possesses a negative or positive electric charge.
- ionic bond
- An electrostatic attraction between oppositely charged ions.
- ionization
- The breaking up of a chemical compound into separate ions.
- isoelectronic
- isomer
- isomerization
- isotope
- A variant of a particular chemical element which differs in the number of neutrons present in the nucleus. All isotopes of a given element have the same number of protons in each atom.
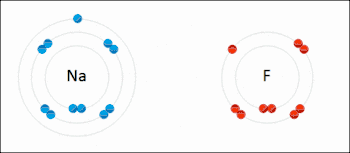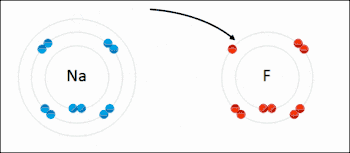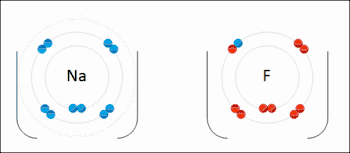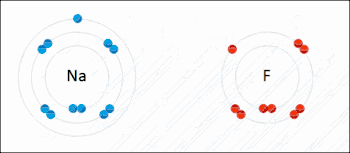
An ionic bond between a sodium atom (Na) and a fluorine atom (F). The sodium atom loses its sole valence electron (leaving the atom with a positive electrical charge), and the fluorine atom gains this same electron via an exothermic process (giving the atom a negative electrical charge). The oppositely charged ions are then attracted to each other to form a new compound called sodium fluoride.
J
K
- kelvin
- The SI base unit of temperature (symbol: K). The Kelvin scale is an absolute thermodynamic temperature scale that uses absolute zero as its null point.
- ketone
- An organic compound with a carbonyl group between two carbon atoms.
- kinetics
- A subfield of chemistry specializing in reaction rates.
- kinetic energy
- The energy of an object due to its motion.
L
- lanthanides
- The periodic series of metallic elements with atomic numbers 57 through 71, from lanthanum through lutetium. Also called lanthanoids.
- lattice
- The unique arrangement of atoms or molecules in a crystalline liquid or solid.
- lattice energy
- law of conservation of energy
- law of conservation of mass
- law of multiple proportions
- laws of thermodynamics
- leveling effect
- Lewis acid
- Lewis base
- Lewis structure
- ligand
- light
- The portion of the electromagnetic spectrum which is visible to the unaided human eye. Also referred to as visible light.
- liquefaction
- liquid
- One of the four fundamental states of matter, characterized by nearly incompressible fluid particles that retain a definite volume but no fixed shape.
- London dispersion forces
- A type of weak intermolecular force.
- Newton's laws of motion
- An object in motion stays in motion; an object at rest stays at rest unless an unbalanced force acts upon it.
M
- magnetic quantum number
- malleability
- See ductility.
- manometer
- An instrument used to measure pressure invented by Evangelista Torricelli in 1643.
- mass
- A property of physical matter that is a measure of its resistance to acceleration when a net force is applied. The SI base unit for mass is the kilogram (kg).
- mass concentration
- mass fraction
- mass number
- mass spectrometry
- matter
- Any substance that has mass and takes up space by having volume.
- metal
- Any chemical element which is a good conductor of both electricity and heat and which readily forms cations and ionic bonds with non-metals.
- melting
- The phase transition of a substance from a solid to a liquid.
- melting point
- metalloid
- A chemical element or substance possessing properties of both metals and non-metals.
- methylene blue
- A heterocyclic aromatic compound with the molecular formula C16H18N3SCl.
- microcentrifuge tube
- A small plastic container that is used to store small amounts of liquid.
- mineral
- miscibility
- mixture
- A material made up of two or more different substances which are mixed physically but are not combined chemically (i.e. a chemical reaction has not taken place which has changed the molecules of the substances into new substances).
- moiety
- molality
- molar attenuation coefficient
- molar mass
- molarity
- mole
- A measurement of an amount of substance in terms of the absolute number of units or entities composing the substance; a single mole contains approximately 6.022×1023 units or entities. Abbreviated mol.
- molecular formula
- molecular orbital
- The region where an electron can be found in a molecule (as opposed to an atom).
- molecular orbital diagram
- molecule
- A number of atoms that are chemically bonded together and collectively electrically neutral.
N
- natural abundance
- neat
- conditions with a liquid reagent or gas performed with no added solvent or cosolvent
- neutron
- A neutral unit or subatomic particle that has no net charge.
- neutrino
- a particle that can travel at speeds close to the speed of light and are created as a result of radioactive decay
- nitrogen
- nucleus
- The centre of an atom, made up of neutrons and protons and possessing a net positive electric charge.
- noble gas
- Any of the six non-metallic elements of Group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). All of the noble gases have outer electron shells that are completely filled in their naturally occurring states, giving them very low chemical reactivity.
- non-metal
- Any element which is not a metal.
- nuclear
- Of or pertaining to the atomic nucleus.
- nuclear chemistry
- The branch of chemistry that studies the various processes and properties relevant to atomic nuclei, including radioactivity.
- nuclear magnetic resonance spectroscopy
- A technique that exploits the magnetic properties of certain nuclei, useful for identifying unknown compounds. Nuclear magnetic resonance is often abbreviated NMR.
- nuclear transmutation
- nuclide
- A species of atom characterized by its mass number, atomic number, and nuclear energy state, provided that the mean life in that state is long enough to be observable.
- number density
- A measure of the concentration of countable objects (atoms, molecules, etc.) in space, expressed as the number per unit volume.
O
- octet rule
- A classical rule for describing the electron configuration of atoms in certain molecules: the maximum number of electron pairs that can be accommodated in the valence shell of an element in the first row of the periodic table is four (or eight total electrons). For elements in the second and subsequent rows, there are many exceptions to this rule. Also called the Lewis octet rule.
- optical activity
- orbital
- may refer to either an atomic orbital or a molecular orbital
- orbital hybridisation
- order of reaction
- organic acid
- organic compound
- Any chemical compound that contains carbon. Compare inorganic compound.
- organic chemistry
- A branch of chemistry concerned with the chemical properties and reactions of organic compounds. Compare inorganic chemistry.
- organic redox reaction
- osmotic pressure
- other metal
- metallic elements in the p-block, characterized by having a combination of relatively low melting points (all less than 950 K) and relatively high electronegativity values (all more than 1.6, revised Pauling).
- oxidation
- oxidation state
- 1) The degree of oxidation (loss of electrons) of an individual atom in a chemical compound; 2) the hypothetical electric charge (positive, negative, or zero) that the atom would have if all bonds to atoms of different elements were 100% ionic, with no covalent component. Also called oxidation number.
- oxidizing agent
- oxoacid
- 1) Any acid having oxygen in the acidic group; 2) any compound which contains oxygen, at least one other element, and at least one hydrogen atom bound to oxygen, and which produces a conjugate base by loss of positive hydrogen ions. Also called an oxyacid or oxacid.
- oxygen
P
- p-block
- pH
- A measure of acidity or basicity of a solution.
- pascal
- passivation
- phase
- phase transition
- 1) A transformation between solid, liquid, and gaseous states of matter and, in rare cases, plasma; 2) the measurable values of the external conditions at which such a transformation occurs.
- phi bond
- physical chemistry
- pi bond
- pipette
- plasma
- One of the four fundamental states of matter, in which very high-energy particles are partially or fully ionized to the point that they display unique properties and behaviors unlike those of the other three states. Plasma does not exist freely on the Earth's surface under natural conditions.
- period
- One of the horizontal rows of the periodic table and the elements it contains.
- periodic table of the elements
- A tabular arrangement of the chemical elements organized by their atomic number, electron configuration, and other chemical properties, whose adopted structure shows periodic trends and is used by chemists to derive relationships between various elements as well as predict the properties and behaviors of undiscovered or newly synthesized elements. The first periodic table of the elements was published by Russian chemist Dmitri Mendeleev in 1869. Also simply called the periodic table.
- polarity
- potential energy
- The stored energy in a body or in a system due to its position in a force field or due to its configuration.
- precipitate
- The formation of a solid in a solution or inside another solid during a chemical reaction or by diffusion in a solid.
- precision
- How close the results of multiple experimental trials are. Compare accuracy.
- photon
- A carrier of electromagnetic radiation of all wavelengths (such as gamma rays and radio waves).
- polyatomic ion
- A molecule composed of two or more covalently bonded atoms which collectively bear a net electric charge and therefore act as an ion.
- proton
- A subatomic particle with a positive electric charge that is found in the nucleus of an atom. Often denoted with the symbol H+.
- protonation
- The addition of a proton (H+) to an atom, molecule, or ion.
- pyrolysis
- The thermal decomposition of materials at elevated temperatures in an inert atmosphere such as a vacuum gas.
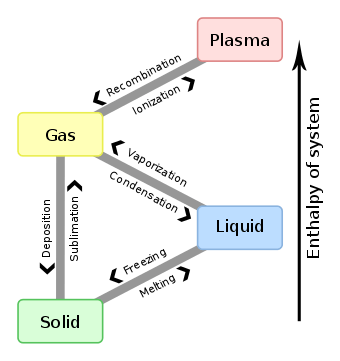
This diagram shows the nomenclature used for each of the different phase transitions
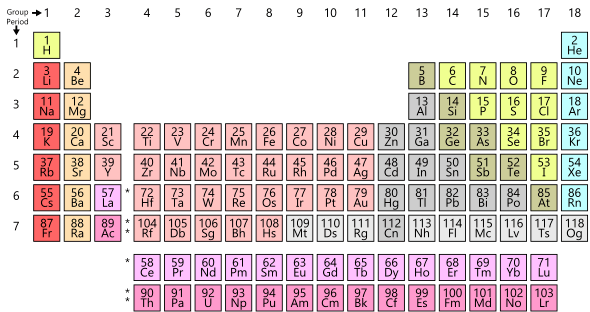
The modern periodic table of the elements. The rows are called periods and the columns are called groups or families.
Q
- quantum mechanics
- The study of how atoms, molecules, subatomic particles, etc. behave and are structured.
- quark
- An elementary particle and a fundamental constituent of matter.
- quantum
- The minimum amount of bundle of energy. Plural quanta.
R
- racemate
- An equimolar mixture of a pair of enantiomers which does not exhibit optical activity. The chemical name or formula of a racemate is distinguished from those of the enantiomers by the prefix (±)- or by the symbols RS and SR.
- radiation
- Energy released in the form of waves or subatomic particles when there is a change from high-energy to low-energy states.
- radioactive decay
- The process of an unstable atomic nucleus losing energy by emitting radiation.
- Raoult's law
- rare-earth metal
- rate equation
- rate law
- reactant
- A substance that is consumed in the course of a chemical reaction. Also called a reagent.
- reaction mechanism
- reaction rate
- The speed at which reactants are converted into products in a chemical reaction.
- reaction rate constant
- reactive intermediate
- reactivity series
- reagent
- A test substance that is added to a system in order to bring about a chemical reaction or to see whether a reaction occurs. Also another name for a reactant.
- redox
- reducing agent
- reduction potential
- resonance
- retort
- rust
S
- s-block
- The collective name for the elements in Groups 1 and 2 of the periodic table (the alkali and alkaline metals), as well as hydrogen and helium.
- salt
- Any ionic compound composed of one or more anions and one or more cations.
- salt bridge
- A device used to connect reduction with oxidation half-cells in an electrochemical cell.
- saline solution
- A common term for a solution of sodium chloride (NaCl) dissolved in water (H2O).
- Schrödinger equation
- A quantum state equation which represents the behaviour of an election around an atom.
- scientific theory
- second-order reaction
- semiconductor
- An electrically conductive solid whose degree of conductivity lies somewhere between that of a conductor and that of an insulator.
- serial dilution
- side chain
- single bond
- A chemical bond that involves the sharing of one pair of electrons.
- sol
- A suspension of solid particles in liquid. Artificial examples include sol-gels.
- solid
- One of the four fundamental states of matter, characterized by relatively low-energy particles packed closely together in rigid structures with definite shape and volume. See Young's modulus.
- solubility
- The property of a solid, liquid, or gaseous solute to dissolve in a solid, liquid, or gaseous solvent. It is typically expressed as the proportion of solute dissolved in the solvent in a saturated solution.
- solute
- The part of a solution that is dissolved into the solvent (for example, sodium chloride (NaCl) is the solute in a solution of saline water).
- solution
- A homogeneous mixture made up of multiple substances generally referred to as solutes and solvents.
- solvation shell
- solvent
- The part of a solution that dissolves the solute (for example, H2O is the solvent in a solution of saline water).
- spectrochemistry
- spectroscopy
- The study of radiation and matter, such as X-ray absorption and emission spectroscopy.
- speed of light
- A physical constant defined as the speed of anything that has zero rest mass (Energyrest = mc², where m is the mass and c is the speed of light).
- standard conditions of temperature and pressure
- A standardisation of ambient temperature and pressure used in order to easily compare experimental results. Standard temperature is 25 degrees Celsius (°C) and standard pressure is 100.000 kilopascals (kPa). Standard conditions are often denoted with the abbreviation STP or STAP.
- state of matter
- matter having a homogeneous, macroscopic phase; gas, plasma, liquid, and solid are the most well-known (in increasing concentration).
- stereochemistry
- stereoisomer
- An isomer which possesses an identical chemical composition but which differs in the spatial arrangement of its atoms.
- stoichiometry
- structural formula
- structural isomer
- sublimation
- The phase transition of a substance from a solid to a limewater fuel or gas without an apparent transition to a liquid in the process.
- subatomic particle
- Any particle that is smaller than an atom. Examples include protons, neutrons and electrons.
- substance
- A material with a definite chemical composition.
- substituent
T
- talc
- A mineral representing the one on the Mohs Scale and composed of hydrated magnesium silicate with the chemical formula H2Mg3(SiO3)4 or Mg3Si4O10(OH)2.
- tarnish
- temperature
- A proportional measure of the average kinetic energy of the random motions of the constituent microscopic particles of a system. The SI base unit for temperature is the kelvin.
- theoretical yield
- See yield.
- theory
- An experimentally testable model describing the nature of a phenomenon.
- thermal conductivity
- The property of a material that allows it to conduct thermal energy or heat (a quantity often denoted by ).
- thermochemistry
- The study of the absorption or release of heat during a chemical reaction.
- thermodynamics
- The study of the effects of changing temperature, volume or pressure (or work, heat, and energy) on a macroscopic scale.
- thermodynamic stability
- The condition of a system being in its lowest energy state with its environment (equilibrium).
- thermometer
- An instrument used to measure temperature.
- titration
- The process of titrating one solution with another. Also called volumetric analysis.
- torr
- A unit for measuring pressure (1 torr is equivalent to 133.322 Pa or 1.3158×10−3 atm).
- transition metal
- An element whose atoms naturally occur with incompletely filled "d" sub-shells. These elements are grouped as the d-block elements in the periodic table.
- transuranic element
- Any element with an atomic number greater than 92. None of the transuranic elements are stable in natural conditions.
- triple bond
- A chemical bond that involves the covalent sharing of three pairs of electrons (for example, the diatomic nitrogen molecule, N2, is composed of two nitrogen atoms linked by a triple bond).
- triple point
- The place where temperature and pressure of three phases are the same (water has a special phase diagram).
- Tyndall effect
- The effect of light scattering by colloidal (mixture where one substance is dispersed evenly through another) or suspended particles.
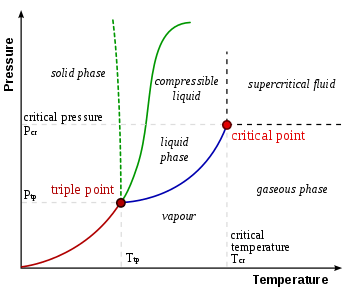
A phase diagram showing the triple point and critical point of a substance
U
- UN number
- A four-digit code used to note hazardous and flammable substances.
- uncertainty
- The notion that any measurement that involves estimation of any amount cannot be exactly reproducible.
- uncertainty principle
- knowing the location of a particle makes the momentum uncertain, while knowing the momentum of a particle makes the location uncertain.
- unified atomic mass unit
- A unit of mass approximately equal to the mass of one proton or neutron and denoted with the symbol u; also called a Dalton and denoted with the symbol Da. Sometimes equated with the technically distinct and obsolete atomic mass unit and abbreviated amu.
- unit cell
- The smallest repeating unit of a lattice.
- unit factor
- statements used in converting between units.
- unpaired electron
V
- vacuum flask
- An insulating storage vessel that can greatly lengthen the time over which its contents remain warmer or cooler than the vessel's ambient environment. Also called a Dewar flask or thermos.
- valence electron
- Any of the outermost electrons of an atom, which are located in electron shells.
- valence bond theory
- A theory explaining the chemical bonding within molecules by discussing valencies, the number of chemical bonds formed by an atom.
- valency
- The combining capacity of an element.
- van der Waals force
- One of the forces (attraction/repulsion) between molecules.
- van 't Hoff factor
- The ratio of moles of particles in solution to moles of solute dissolved.
- vapor
- When a substance is below the critical temperature while in the gas phase.
- vapor pressure
- The pressure of vapor over a liquid at equilibrium.
- vaporization
- The phase transition of a substance from a liquid to a gas. Also called boiling.
- viscosity
- A measure of the resistance of a liquid to flow.
- volt
- A unit that measures the electrical potential transferred. One volt (V) is defined as and equivalent to one Joule of work per coulomb.
- voltmeter
- An instrument that measures electrical cell potential.
- volume
- The quantity of three-dimensional space enclosed by a closed surface, or the space that a substance (solid, liquid, gas, or plasma) or shape occupies or contains. The SI unit for volume is the cubic metre (m3).
- volumetric analysis
- See titration.
- volumetric flask
W
- watch glass
- water
- A polar inorganic compound with the chemical formula H2O that is a tasteless, odorless, and generally colorless liquid at standard temperature and pressure, though it also occurs naturally as a solid and a gas at the Earth's surface. It is the most abundant substance on Earth and therefore an integral component of virtually all chemical and biological systems. Water is often described as the "universal solvent" for its inherent ability to dissolve many substances.
- wave function
- a function describing the electron's position in a three-dimensional space.
- work
- the amount of force over distance and is in terms of joules (energy).
X
- X-ray
- A form of ionizing, electromagnetic radiation between gamma and UV rays in the electromagnetic spectrum.
- X-ray diffraction
- a method for establishing structures of crystalline solids using single wavelength X-rays and looking at diffraction pattern.
- X-ray photoelectron spectroscopy
- A spectroscopic technique used to measure the composition of a material.
Y
- yield
- The quantifiable amount of product produced during a chemical reaction.
Z
- zone melting
- A way to remove impurities from an element by melting it and slowly travel down an ingot (cast).
- zwitterion
- A chemical compound whose net charge is zero and hence is electrically neutral. But there are some positive and negative charges in it, due to the formal charge, owing to the partial charges of its constituent atoms.
- zinc
- A metallic chemical element with atomic number 30 and symbol Zn.
See also
External links
| Wikibooks has a book on the topic of: Chemistry |
| Wikiquote has quotations related to: English_chemistry_mnemonics |
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.