Gattermann reaction
| Gattermann formylation | |
|---|---|
| Named after | Ludwig Gattermann |
| Reaction type | Substitution reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000139 |
The Gattermann reaction, (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) in the presence of a Lewis acid catalyst such as AlCl3. It is named for the German chemist Ludwig Gattermann[1] and is similar to the Friedel–Crafts reaction.
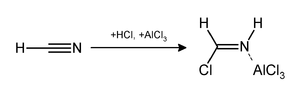
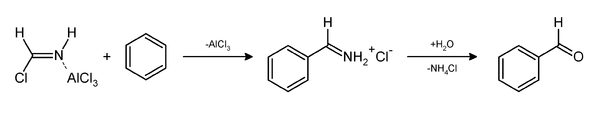
The reaction can be simplified by replacing the HCN/AlCl3 combination with zinc cyanide.[2] Although it is also highly toxic, Zn(CN)2 is a solid, making it safer to work with than gaseous HCN.[3] The Zn(CN)2 reacts with the HCl to form the key HCN reactant and Zn(CN)2 that serves as the Lewis-acid catalyst in-situ. An example of the Zn(CN)2 method is the synthesis of mesitaldehyde from mesitylene.[4]
Gattermann–Koch reaction
| Gattermann–Koch formylation | |
|---|---|
| Named after | Ludwig Gattermann Julius Arnold Koch |
| Reaction type | Substitution reaction |
The Gattermann–Koch reaction, named after the German chemists Ludwig Gattermann and Julius Arnold Koch,[5] is a variant of the Gattermann reaction in which carbon monoxide (CO) is used instead of hydrogen cyanide.[6]
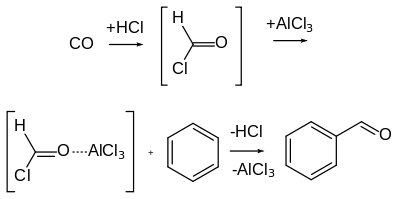
Unlike the Gattermann reaction, this reaction is not applicable to phenol and phenol ether substrates.[3] Additionally, when zinc chloride is used as the catalyst, the presence of traces of copper(I) chloride co-catalyst is often necessary.
See also
References
- ↑ Gattermann, L.; Berchelmann, W. (1898). "Synthese aromatischer Oxyaldehyde". Berichte der deutschen chemischen Gesellschaft. 31 (2): 1765–1769. doi:10.1002/cber.18980310281.
- ↑ Adams R.; Levine, I. (1923). "Simplification of the Gattermann Synthesis of Hydroxy Aldehydes". J. Am. Chem. Soc. 45 (10): 2373–77. doi:10.1021/ja01663a020.
- 1 2 Adams, Roger (1957). Organic Reactions, Volume 9. New York: John Wiley & Sons, Inc. pp. 38 & 53–54. doi:10.1002/0471264180.or009.02. ISBN 9780471007265.
- ↑ Fuson, R. C.; Horning, E. C.; Rowland, S. P.; Ward, M. L. (1955). "Mesitaldehyde". Organic Syntheses. doi:10.15227/orgsyn.023.0057. ; Collective Volume, 3, p. 549
- ↑ Gattermann, L.; Koch, J. A. (1897). "Eine Synthese aromatischer Aldehyde". Chemische Berichte. 30: 1622. doi:10.1002/cber.18970300288.
- ↑ Li, Jie Jack (2003). Name Reactions: A Collection of Detailed Reaction Mechanisms (available on Google Books) (2nd ed.). Springer. p. 157. ISBN 3-540-40203-9.