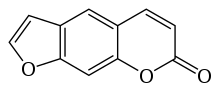
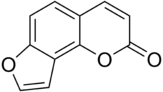
Furanocoumarin
The furanocoumarins, or furocoumarins, are a class of organic chemical compounds produced by a variety of plants. They are biosynthesized partly through the phenylpropanoid pathway and the mevalonate pathway, which is biosynthesized by a coupling of dimethylallyl pyrophosphate (DMAPP) and 7-hydroxycoumarin (umbelliferone).
Structure
The chemical structure of furanocoumarins consists of a furan ring fused with coumarin. The furan may be fused in different ways producing several isomers. The compounds that form the core structure of the two most common isomers are psoralen and angelicin. Derivatives of these two core structures are referred to respectively as linear and angular furanocoumarins.[1]
Effects
Direct toxicity
Many furanocoumarin compounds are toxic. The phytochemicals enter the nucleus of epithelial cells and form a bond with the DNA, which causes the cells to die. As a result, the skin is unable to protect itself from sunlight, which leads to phytophotodermatitis, a serious skin inflammation.
There is some speculation that perhaps furanocoumarins are produced by plants as a defense mechanism against predators such as insects and mammals.[2] What's more likely, however, is that furanocoumarins are related to a plant's natural defense against fungal attack.[3] In particular, the linear furanocoumarins (psoralen, bergapten, and methoxsalen), which occur naturally in Apiaceae, Rutaceae, and other plant families, are known to be toxic to fungi[4] whereas plants that cause phytophotodermatitis usually contain linear furanocoumarins.[3]
Furanocoumarins are found in the sap of plants such as Ammi majus, parsnip, and giant hogweed. At least 36 species of the Heracleum genus in the Apiaceae family are known to contain one or more furanocoumarin compounds.[5]
Medication interactions
Furanocoumarins have other biological effects as well. For example, in humans, bergamottin and 6',7'-dihydroxybergamottin are responsible for the "grapefruit juice effect", in which these furanocoumarins affect certain P450 liver and gut enzymes, such as the inhibition of CYP3A4 which either activates or deactivates many drugs, thus leading to higher or lower levels in the bloodstream.[6] Furanocoumarins have various effects which can specifically increase or decrease (depending on the drug) the blood levels of many pharmaceuticals in ways that can be life-threatening and so FDA approved drugs will include warnings for grapefruit.
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "furanocoumarins". IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "furocoumarins".
- ↑ Berenbaum, May (June 14, 2010). "Furanocoumarins as potent chemical defenses".
- 1 2 McGovern, Thomas W; Barkley, Theodore M. "Phytophotodermatitis". The Electronic Textbook of Dermatology--Botanical Dermatology. Retrieved August 11, 2018.
- ↑ Camm, E. L.; Wat, C. K.; Towers, G. H. N. (1976). "An assessment of the roles of furanocoumarins in Heracleum lanatum". Can. J. Bot. 54 (22): 2562--2566.
- ↑ Mitchell, John; Rook, Arthur (1979). Botanical Dermatology: Plants and Plant Products Injurious to the Skin. Vancouver: Greengrass. pp. 692–699.
- ↑ Kakar, SM; Paine, MF; Stewart, PW; Watkins, PB (2004). "6',7'-Dihydroxybergamottin contributes to the grapefruit juice effect". Clinical Pharmacology and Therapeutics. 75 (6): 569–579. doi:10.1016/j.clpt.2004.02.007. PMID 15179411.