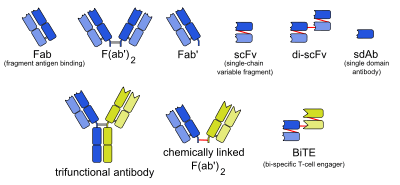
Fragment antigen-binding
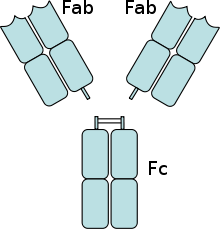
The antigen-binding (Fab) fragment is a region on an antibody that binds to antigens. It is composed of one constant and one variable domain of each of the heavy and the light chain. The variable domain contains the paratope (the antigen-binding site), comprising a set of complementarity determining regions, at the amino terminal end of the monomer. Each arm of the Y thus binds an epitope on the antigen.
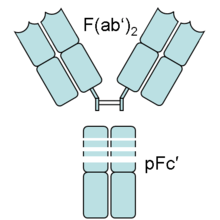
In an experimental setting, Fc and Fab fragments can be generated in the laboratory. The enzyme papain can be used to cleave an immunoglobulin monomer into two Fab fragments and an Fc fragment. The enzyme pepsin cleaves below the hinge region, so a F(ab')2 fragment and a pFc' fragment is formed. Recently another enzyme for generation of F(ab')2 has been commercially available. The enzyme IdeS (Immunoglobulin degrading enzyme from Streptococcus pyogenes, trade name FabRICATOR) cleaves IgG in a sequence specific manner at neutral pH. The F(ab')2 fragment can be split into two Fab' fragments by mild reduction.[1]
The variable regions of the heavy and light chains can be fused together to form a single-chain variable fragment (scFv), which is only half the size of the Fab fragment, yet retains the original specificity of the parent immunoglobulin.[2]

See also
References
- ↑ Larsson, Lars-Inge (September 1988). Immunocytochemistry: Theory and practice. Crc Press. p. 1. ISBN 0-8493-6078-1.
- ↑ Janeway, CA, Jr.; et al. (2001). Immunobiology (5th ed.). Garland Publishing. ISBN 0-8153-3642-X.