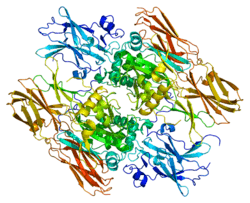
Coagulation factor XIII, A1 polypeptide
Coagulation factor XIII A chain is a protein that in humans is encoded by the F13A1 gene.
Function
This gene encodes the coagulation factor XIII A subunit. Coagulation factor XIII is the last zymogen to become activated in the blood coagulation cascade. Plasma factor XIII is a heterotetramer composed of 2 A subunits and 2 B subunits. The A subunits have catalytic function, and the B subunits do not have enzymatic activity and may serve as plasma carrier molecules. Platelet factor XIII is composed of just 2 A subunits, which are identical to those of plasma origin. Upon cleavage of the activation peptide by thrombin and in the presence of calcium ion, the plasma factor XIII dissociates its B subunits and yields the same active enzyme, factor XIIIa, as platelet factor XIII. This enzyme acts as a transglutaminase to catalyze the formation of gamma-glutamyl-epsilon-lysine crosslinking between fibrin molecules, thus stabilizing the fibrin clot. It also crosslinks alpha-2-plasmin inhibitor, or fibronectin, to the alpha chains of fibrin. Factor XIII deficiency is classified into two categories: type I deficiency, characterized by the lack of both the A and B subunits; and type II deficiency, characterized by the lack of the A subunit alone. These defects can result in a lifelong bleeding tendency, defective wound healing, and habitual abortion.[5]
Interactions
Coagulation factor XIII, A1 polypeptide has been shown to interact with F13B.[6][7]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000124491 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000039109 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ "Entrez Gene: F13A1 coagulation factor XIII, A1 polypeptide".
- ↑ Carrell NA, Erickson HP, McDonagh J (Jan 1989). "Electron microscopy and hydrodynamic properties of factor XIII subunits". J. Biol. Chem. 264 (1): 551–6. PMID 2491853.
- ↑ Achyuthan KE, Rowland TC, Birckbichler PJ, Lee KN, Bishop PD, Achyuthan AM (Sep 1996). "Hierarchies in the binding of human factor XIII, factor XIIIa, and endothelial cell transglutaminase to human plasma fibrinogen, fibrin, and fibronectin". Mol. Cell. Biochem. 162 (1): 43–9. doi:10.1007/bf00250994. PMID 8905624.
Further reading
- Adány R (1997). "Intracellular factor XIII: cellular distribution of factor XIII subunit a in humans". Semin. Thromb. Hemost. 22 (5): 399–408. doi:10.1055/s-2007-999038. PMID 8989823.
- Adány R, Bárdos H (2003). "Factor XIII subunit A as an intracellular transglutaminase". Cell. Mol. Life Sci. 60 (6): 1049–60. doi:10.1007/s00018-003-2178-9. PMID 12861374.
- Bereczky Z, Katona E, Muszbek L (2005). "Fibrin stabilization (factor XIII), fibrin structure and thrombosis". Pathophysiol. Haemost. Thromb. 33 (5–6): 430–7. doi:10.1159/000083841. PMID 15692256.
- Board P, Coggan M, Miloszewski K (1992). "Identification of a point mutation in factor XIII A subunit deficiency". Blood. 80 (4): 937–41. PMID 1353995.
- Kamura T, Okamura T, Murakawa M, Tsuda H, Teshima T, Shibuya T, Harada M, Niho Y (1992). "Deficiency of coagulation factor XIII A subunit caused by the dinucleotide deletion at the 5' end of exon III". J. Clin. Invest. 90 (2): 315–9. doi:10.1172/JCI115864. PMC 443104. PMID 1644910.
- Harsfalvi J, Fesus L, Tarcsa E, Laczko J, Loewy AG (1991). "The presence of a covalently cross-linked matrix in human platelets". Biochim. Biophys. Acta. 1073 (2): 268–74. doi:10.1016/0304-4165(91)90131-Y. PMID 2009280.
- Bishop PD, Teller DC, Smith RA, Lasser GW, Gilbert T, Seale RL (1990). "Expression, purification, and characterization of human factor XIII in Saccharomyces cerevisiae". Biochemistry. 29 (7): 1861–9. doi:10.1021/bi00459a028. PMID 2184890.
- Hilgenfeld R, Liesum A, Storm R, Metzner HJ, Karges HE (1990). "Crystallization of blood coagulation factor XIII by an automated procedure". FEBS Lett. 265 (1–2): 110–2. doi:10.1016/0014-5793(90)80896-Q. PMID 2365049.
- Carrell NA, Erickson HP, McDonagh J (1989). "Electron microscopy and hydrodynamic properties of factor XIII subunits". J. Biol. Chem. 264 (1): 551–6. PMID 2491853.
- Takahashi N, Takahashi Y, Putnam FW (1986). "Primary structure of blood coagulation factor XIIIa (fibrinoligase, transglutaminase) from human placenta". Proc. Natl. Acad. Sci. U.S.A. 83 (21): 8019–23. doi:10.1073/pnas.83.21.8019. PMC 386858. PMID 2877456.
- Grundmann U, Amann E, Zettlmeissl G, Küpper HA (1986). "Characterization of cDNA coding for human factor XIIIa". Proc. Natl. Acad. Sci. U.S.A. 83 (21): 8024–8. doi:10.1073/pnas.83.21.8024. PMC 386859. PMID 2877457.
- Ichinose A, Davie EW (1988). "Characterization of the gene for the a subunit of human factor XIII (plasma transglutaminase), a blood coagulation factor". Proc. Natl. Acad. Sci. U.S.A. 85 (16): 5829–33. doi:10.1073/pnas.85.16.5829. PMC 281858. PMID 2901091.
- Board PG, Webb GC, McKee J, Ichinose A (1988). "Localization of the coagulation factor XIII A subunit gene (F13A) to chromosome bands 6p24----p25". Cytogenet. Cell Genet. 48 (1): 25–7. doi:10.1159/000132580. PMID 2903011.
- Ichinose A, Hendrickson LE, Fujikawa K, Davie EW (1987). "Amino acid sequence of the a subunit of human factor XIII". Biochemistry. 25 (22): 6900–6. doi:10.1021/bi00370a025. PMID 3026437.
- Takagi T, Doolittle RF (1974). "Amino acid sequence studies on factor XIII and the peptide released during its activation by thrombin". Biochemistry. 13 (4): 750–6. doi:10.1021/bi00701a018. PMID 4811064.
- Yee VC, Pedersen LC, Bishop PD, Stenkamp RE, Teller DC (1995). "Structural evidence that the activation peptide is not released upon thrombin cleavage of factor XIII". Thromb. Res. 78 (5): 389–97. doi:10.1016/0049-3848(95)00072-Y. PMID 7660355.
- Coggan M, Baker R, Miloszewski K, Woodfield G, Board P (1995). "Mutations causing coagulation factor XIII subunit A deficiency: characterization of the mutant proteins after expression in yeast". Blood. 85 (9): 2455–60. PMID 7727776.
- Kaczmarek E, Liu Y, Berse B, Chen CS, McDonagh J (1995). "Biosynthesis of plasma factor XIII: evidence for transcription and translation in hepatoma cells". Biochim. Biophys. Acta. 1247 (1): 127–34. doi:10.1016/0167-4838(94)00167-f. PMID 7873582.
- Yee VC, Pedersen LC, Le Trong I, Bishop PD, Stenkamp RE, Teller DC (1994). "Three-dimensional structure of a transglutaminase: human blood coagulation factor XIII". Proc. Natl. Acad. Sci. U.S.A. 91 (15): 7296–300. doi:10.1073/pnas.91.15.7296. PMC 44386. PMID 7913750.
- Suzuki K, Iwata M, Ito S, Matsui K, Uchida A, Mizoi Y (1994). "Molecular basis for subtypic differences of the "a" subunit of coagulation factor XIII with description of the genesis of the subtypes". Hum. Genet. 94 (2): 129–35. doi:10.1007/bf00202857. PMID 7913909.